Abstract
Background: Finding the best therapy option to treat myelofibrosis (MF) represents a huge challenge. Ruxolitinib (R) is a potent JAK1/2 inhibitor that has demonstrated improved survival and symptomatology in MF patients. There are a lot researches looking for the best combination of JAK inhibitor to improve its efficacy. In a previous work, we screened different drugs to evaluate if they were synergistic with ruxolitinib, we found that ruxolitinib was synergistic with nilotinib (N) and Prednisolone (P) as well as other drugs including bortezomib and HSP-90 inhibitors.
Aim: To study the synergistic behavior and mechanism of actions of ruxolitinib in combination with nilotinib and prednisolone both in patient samples and cell lines.
Methods: We have studied 20 secondary or primary MF patients and cell line: BA/F3 transfected with mutated JAK2 V617F (BA/F3 JAK2V617F).
Classical assay was performed for patient samples: mononuclear cells isolated from peripheral blood were cultured during 2 weeks in Methocult TM GF_H4535 with 20 ng/ml IL-3, and 50 ng/ml SCF, in presence of increasing concentrations of R, N or P or their combinations.
Also, mononuclear cells were cultured as described above, during 2 weeks but without drugs. Then, cells were washed with PBS and cultured 72 hours in RPMI 10% FBS and plated at 15,000 per well in 96-well plates with increasing concentrations of drugs.
After both assay, cells were labeled with Annexin V and CD13 and analyzed in the Exvitech platform, an automated multiparametric flow cytometry platform.
For cell lines, these were cultured in RPMI 10% FBS in the presence of increasing concentration of drugs. After 48 h of incubation, we performed a wst-8 assay to evaluate cell viability.
To analyzed effect of treatment on survival and proliferation signaling pathways, cell lines were treated for 30 min with R 0.032 µM, N 1.6 µM and P 0.8 µM or combinations and total lysates were collected. To perform western blot, we use phospho-STAT5 (Tyr 964) and STAT5 after stripping as primary antibody and β-actin as load control.
Graphpad Prism or XLFit was used to analyze dose-response curves. Synergism will be evaluated by the Median Effect methods described by T-C Chou and P. Talalay.
Results:
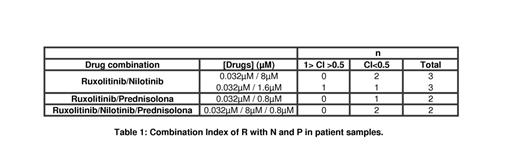
In patient samples when performed the classical assay, we found synergistic interaction in all combination in most of the patients (Table 1). Briefly, all combinations showed synergetic behavior (C<1) and triple combination were strongly synergetic in all patient samples: CI = 0.213 and 0.348.
However, when pathologic cells were amplified in a methylcellulose culture and then analyzed, we found a strong potentiation, representing an important dose reduction of R in the presence of N (Dose-Reduction Index (DRI) = 6.10, (n = 4) and a DRI = 110.17 when R is combined with P (n =2). There was not a synergistic interaction between R with N or P, because R was not efficient enough in this model.
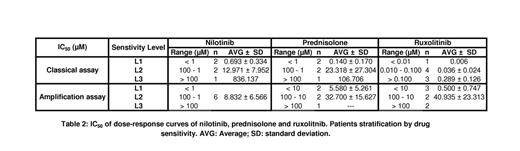
Interestingly, R had more effect in the classical assay, always less than 0.500 µM, than in the amplification assay even on less sensitive patient samples (Table 2). R seems inhibit growth and development of hematopoietic stem cells but not differentiated cells. N had a wide range of action in classical assay, but in amplification assay the effect was homogeneous, around 9 µM. P had similar heterogeneous effect on both assays from high nM to low µM.
The 3 drugs were more potent in cell line BA/F3V617F than in patients: IC50 R = 15 nM; IC50 N = 3.35 µM; IC50 P = 4 nM. All drug combination tested were synergistic (CI<1). We choose [R] = 0.032 µM, [N] = 1.6 µM and [P] = 0.8 µM and their combinations to analyzed the signaling pathways by western blot because of their strong synergistic behavior: CI RN = 0.144; CI RP = 0.178; CI RNP = 0.223. We studied the effect of the different treatment in the phosphorylation on STAT5 after 30 min of incubation with drugs. We observed a decreased of phosphorylated form in R treatment and combination with N and P but not in N or P alone. The maximal effect was found when RNP combination was employed.
Conclusions: we have showed that ruxolitinib have a synergistic effect with nilotinib and prednisone in MF patient samples. This effect is in part mediated through a greatest inhibition of STAT5 pathway. This preclinical study warranties a clinical trial with this combination in patients with MF.
Hernandez-Campo:Vivia Biotech: Employment. Gorrochategui:Vivia Biotech: Employment. Primo:Vivia Biotech: Employment. Ballesteros:Vivia Biotech: Employment, Equity Ownership. Martinez-Lopez:Vivia Biotech: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.