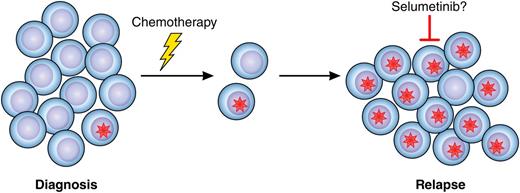
In this issue of Blood, Irving et al report a high frequency of rat sarcoma (RAS) signaling pathway mutations in relapsed childhood B-cell precursor acute lymphoblastic leukemia (BCP ALL) and present detailed data on the clonal evolution of RAS mutation-positive blasts from diagnosis to first, and, in some cases, second relapse.1 This study is important for 2 reasons; first because it adds to our understanding of the complex genetic mechanisms involved in chemoresistant ALL and second because the authors also present data from in vitro and in vivo experiments indicating that the mitogen-activated protein kinase kinase (MEK) inhibitor selumetinib could be a novel treatment option in relapsed ALL with activation of the RAS pathway (see figure).
Mutations in the RAS signaling pathway (red star) are frequently present in a small subset of leukemic blast cells at diagnosis of pediatric ALL. Cells harboring the mutation may survive chemotherapy, resulting in relapse, which could be treatable with selumetinib, targeting MEK downstream of RAS signaling. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mutations in the RAS signaling pathway (red star) are frequently present in a small subset of leukemic blast cells at diagnosis of pediatric ALL. Cells harboring the mutation may survive chemotherapy, resulting in relapse, which could be treatable with selumetinib, targeting MEK downstream of RAS signaling. Professional illustration by Patrick Lane, ScEYEnce Studios.
Although ALL usually displays 1 dominant clone—ie, identical somatic mutations in the majority of blast cells—at diagnosis, next-generation sequencing and xenografts have recently revealed that cases frequently also harbor mutations that are present in only a subset of the cells, indicating a previously unrecognized genetic heterogeneity.2,3 Evidence pointing in the same direction has come from comparisons of ALL at diagnosis and relapse, where microdeletions and mutations present only at relapse have been backtracked to minor subclones in the corresponding diagnostic sample.4,5 Genetic heterogeneity at diagnosis may be one of the underlying factors when the leukemia relapses, because small subclones may survive the treatment that eradicates the main diagnostic clone and subsequently lead to disease recurrence. Irving et al investigate mutations affecting the RAS signaling pathway in samples from 206 relapsed BCP ALLs. Mutations in this pathway are among the most common somatic changes in human malignancies and are found in a wide range of tumors. In pediatric ALL, mutations of the KRAS, NRAS, PTPN11, and FLT3 genes, all involved in the RAS pathway, are seen in ∼35% of cases at diagnosis, with varying frequency in different genetic subtypes.5,6 Although RAS mutations have not been shown to affect prognosis in childhood ALL in general,6 they are a poor prognostic sign in MLL-rearranged ALL.7 Irving et al detect a RAS mutation frequency similar to that seen in diagnostic cases, but in contrast to RAS mutations at diagnosis, they found that mutations at first relapse were associated with dismal prognostic features, such as a higher proportion of early relapses and central nervous system (CNS) involvement. The role of RAS mutations in relapsed ALL is poorly understood, with a handful of reported cases showing mutations both appearing and disappearing at relapse compared with diagnosis.5,8 Irving et al report the largest series to date on RAS involvement at ALL relapse, showing that half of their cases gained the mutation at relapse and extending their study to show that the relapse mutations were frequently present in small subclones in the diagnostic sample, indicating genetic heterogeneity. Furthermore, in cases that had a second relapse, the mutation present at first relapse persisted in 80% of cases, suggesting that RAS mutations are one of the drivers in disease recurrence. In 1 sample, Irving et al were also able to show enrichment of 2 subpopulations with KRAS mutations during induction therapy. Although these data indicate that RAS-mutated blasts have a survival advantage during therapy, it should be noted that RAS mutations were also found in small subclones in diagnostic samples from ALLs that have not subsequently relapsed, confusing the issue of whether RAS mutations per se confer resistance to treatment. However, taken together, their data suggest that mutations resulting in activation of the RAS pathway contribute to relapse in childhood BCP ALL and that relapsed ALL with RAS mutations is associated with a more aggressive disease compared with cases lacking such mutations.
Although pediatric ALL in general has a favorable prognosis, with >80% overall survival rates on contemporary treatment protocols, cases that relapse have dismal outcome and display overall survival rates of only 30% to 40%.9 The high incidence of RAS mutations at relapse and the association with poor prognostic features suggest that targeting the RAS pathway may be a treatment option in relapsed childhood ALL. To test this, Irving et al perform in vitro and in vivo studies on RAS mutation-positive blast cells treated with selumetinib. This drug targets MEK, a downstream effector molecule in the RAS signaling pathway, and has shown promising results in clinical trials for several solid tumors with activation of the pathway, including KRAS-mutated non-small-cell lung cancer.10 Irving et al report that ALL blasts with mutations in the RAS pathway have constitutive activation of the pathway, as ascertained by phosphorylated extracellular signal-regulated kinase (ERK) levels, and that they show a cytotoxic response to selumetinib both in vitro and in vivo in mouse xenografts. Considering that they also detected a high frequency of CNS involvement in the RAS-mutated relapsed ALL cases, it is particularly notable that they found that mice engrafted with an NRAS-mutated ALL displayed a significant reduction in CNS infiltration when treated with selumetinib. Thus, selumetinib is a promising candidate for treating high-risk pediatric ALL with activation of the RAS pathway, such as RAS mutation-positive relapses and MLL-rearranged ALL.
Conflict-of-interest disclosure: The author declares no competing financial interests.