Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) has been considered as the treatment of choice for patients with high-risk chronic lymphocytic leukemia (HR-CLL; ie, refractory to purine analogs, short response [<24 months] to chemoimmunotherapy, and/or presence of del[17p]/TP53 mutations). Currently, treatment algorithms for HR-CLL are being challenged by the introduction of novel classes of drugs. Among them, BCR signal inhibitors (BCRi) and B-cell lymphoma 2 antagonists (BCL2a) appear particularly promising. As a result of the growing body of favorable outcome data reported for BCRi/BCL2a, uncertainty is emerging on how to advise patients with HR-CLL about indication for and timing of HSCT. This article provides an overview of currently available evidence and theoretical considerations to guide this difficult decision process. Until the risks and benefits of different treatment strategies are settled, all patients with HR-CLL should be considered for treatment with BCRi/BCL2a. For patients who respond to these agents, there are 2 treatment possibilities: (1) performing an HSCT or (2) continuing treatment with the novel drug. Individual disease-specific and transplant-related risk factors, along with patient’s preferences, should be taken into account when recommending one of these treatments over the other.
Introduction
In the last decade, important progress has been made in treating patients with chronic lymphocytic leukemia (CLL), with the advent of chemoimmunotherapy being the most important improvement.1-7 Unfortunately, in some patients, the disease is either refractory to the standard treatment or progresses after a short period of time. In such patients, the prognosis is dismal, and allogeneic hematopoietic stem cell transplantation (HSCT) has been regarded as treatment of choice if they are eligible for transplantation. In 2007, a consensus paper identified high-risk CLL (HR-CLL; disease refractory to purine analogs, disease relapsing within 2 years after purine analog combination treatment, and/or disease with del[17p]/TP53 mutations) as a situation in which HSCT should be considered.8 The concept of HR-CLL (also termed “highest-risk CLL” or “ultra-high-risk CLL”9 ) has been widely accepted by the scientific community.10-12
The established treatment algorithms for CLL are currently challenged by novel classes of drugs whose mechanisms of action are different from traditional cytotoxic agents and antibodies. The most promising and best developed of these agents are inhibitors of kinases downstream of the B-cell receptor, such as ibrutinib and idelalisib (BCR signal inhibitors [BCRi]) and the selective B-cell lymphoma 2 antagonist (BCL2a) ABT-199.13-15 Although the available information is limited, preliminary observations strongly suggest that these agents have the potential to modify the standard treatment for CLL, including the role of HSCT.16 However, the mid- and long-term efficacy and toxicity, optimum mode of use (combination partners, treatment line, sequence), and the ultimate impact of new agents on CLL treatment are not yet defined.
As a result of the accumulating favorable outcome data reported for the new drugs, there is concern about whether patients with HR-CLL should continue to be offered HSCT. The objective of this article is to summarize current evidence and theoretical considerations for informing patients with HR-CLL about the potential risks and benefits of transplantation and alternative treatments since the role of the new agents in CLL management is not definitively settled.
Current evidence
What we know about HSCT in HR-CLL
Graft-versus-leukemia activity is effective.
The basis for HSCT in CLL is graft-versus-leukemia (GVL) activity. Evidence for GVL efficacy in CLL derives from the lower relapse risk after chronic graft-versus-host disease (GVHD),17-19 and the higher relapse risk associated with T-cell depletion.20,21 The strongest proof of the GVL principle in CLL comes from studies that analyze minimal residual disease (MRD). MRD kinetics studies after HSCT for HR-CLL demonstrate that MRD clearance often occurs only in the context of chronic GVHD or immune interventions, such as tapering of immunosuppression or donor lymphocyte infusions.17-19,22,23
Long-term disease control and curative potential.
In keeping with the GVL effect, larger studies on reduced-intensity conditioning (RIC) HSCT in CLL show event-free-survival (EFS) and overall survival (OS) rates of 35% to 45% and 50% to 60%, respectively, at 5 years (Table 1). Five-year survival is better in those patients who have sensitive and nonbulky disease, ranging from 54% to 79%.19,24-28 MRD studies consistently indicate that permanent MRD negativity can be reached in up to 50% of patients allografted for HR-CLL,18,19 suggesting that HSCT is capable of curing the disease.
HSCT overcomes the poor prognostic impact of genetic risk factors and fludarabine refractoriness.
Prognostic factors that negatively influence the outcome of CLL under chemoimmunotherapy, such as unmutated IGHV genes, unfavorable genetic abnormalities (del[17p], TP53 mutation), and purine analog refractoriness, do not adversely affect EFS and OS after HSCT.19,24,26,27,31 A complex karyotype (ie, more than 3 genetic lesions) may confer an adverse prognosis in CLL, especially if it includes del(17p), under both chemoimmunotherapy and BCR inhibition.32-34 Only a few studies have investigated whether a complex karyotype has an impact on transplantation outcome with no consistent results so far.27,35
CLL relapse after HSCT does not convey an inevitably dismal prognosis.
Although individual patients who relapse after HSCT can be durably rescued by immunotherapeutic approaches, such as immunosuppression withdrawal or donor lymphocyte infusion,19,24,28,36 most clinical relapses are not sensitive to immune manipulation. These patients, however, can benefit from salvage treatment. With all the necessary caveats that small retrospective studies pose, prognosis of patients with HR-CLL who relapse or progress after HSCT appears to be not necessarily poorer than that of patients with HR-CLL who have not received a transplant.37-39
Early mortality with modern HSCT approaches is low.
Current RIC HSCT strategies are much less toxic than traditional myeloablative conditioning regimens: grade III-IV nausea and mucositis affect only a minority of patients undergoing RIC for CLL, and although severe infections still occur in up to 60% of the patients, only a few result in life-threatening complications. Accordingly, the early death rate of CLL transplants (ie, death within the first 100 days after HSCT) has dramatically decreased from previous rates of up to 40% with traditional conditioning to less than 5% in the most recent studies with RIC (Table 2). The good tolerability of RIC HSCT allows offering the procedure to older patients and patients with comorbidity who represent the bulk of the CLL population, thereby greatly increasing the accessibility of HSCT in CLL.
Nonrelapse mortality mounts up to 15% to 30% within the first 2 years after HSCT.
Despite remarkable improvements regarding early fatalities, nonrelapse mortality (NRM) after RIC HSCT for CLL still occurs in 15% to 30% of patients during the first 2 years posttransplant, mainly because of complications of acute and chronic GVHD (Table 2). There is no consistent information on risk factors for NRM after RIC HSCT in CLL. However, individual studies identified refractory disease at transpant25 and high HSCT comorbidity index (HCT-CI)24 as significant independent predictors for NRM. Results of large studies across common HSCT indications have provided evidence that absence of comorbidity as indicated by a low HCT-CI,40-42 donor-recipient HLA match,43-45 the implementation of an effective quality management system in the transplant center,46 and center experience47 all are associated with a significantly reduced risk of NRM.
Patient-specific variables have to be taken into account when considering the indication of HSCT in CLL.
Patient- and transplant-related factors, such HCT-CI,24,27 age,25,27 and donor HLA match,30 have been shown to influence EFS after HSCT for HR-CLL. Moreover, the European Society for Blood and Marrow Transplantation score, which amalgamates patient-, transplant-, and disease-specific parameters (ie, age, donor, donor-recipient sex combination, time interval from diagnosis to transplant, disease status),48 has also been found to predict the outcome of HSCT for CLL.30
Chronic GVHD will affect quality of life in at least 25% of surviving patients.
Apart from its impact on NRM, chronic GVHD is the major determinant affecting quality of life after HSCT. At least 25% of survivors will experience impaired life satisfaction during the first posttransplant years because of chronic GVHD.49 However, morbidity associated with chronic GVHD may decrease over time.24,25
What we don’t know about HSCT in HR-CLL
Does HSCT change the natural course of poor-risk CLL?
In spite of strong evidence that HSCT overcomes the negative influence of poor prognostic markers (eg, del[17p]/TP53 mutations), it could be argued that this notion has not been validated in randomized studies. However, a recent retrospective study that compared patients with HR-CLL with transplant indication (according to the European Society for Blood and Marrow Transplantation criteria) in a donor-versus-no-donor analysis suggested a survival advantage for patients with an available donor.50 Likewise, a survival advantage for HSCT was also found in a systematic meta-analysis using a Markov decision model.51
Is HSCT effective in patients with a history of Richter’s transformation?
To date there are only 2 small retrospective studies addressing this question. Investigators from the MD Anderson Cancer Center compared 7 patients who underwent allogeneic HSCT in remission with 48 patients with active disease who were allografted, underwent autologous HSCT, or were not transplanted and found a significant survival benefit in the small group of patients allografted in remission.52 Similarly a significantly better OS in 15 patients who underwent HSCT in remission compared with 9 patients with uncontrolled disease has been observed in a registry analysis.53 There is no information on HSCT results based on the clonal origin of Richter’s syndrome or Epstein-Barr virus status.
Is HSCT effective after exposure to BCRi?
There is virtually no information about the potential impact of new drugs on the outcome of subsequent allotransplantation. It is also unknown how effective HSCT can be in patients with CLL who relapse or progress under BCRi/BCL2a.
Will HSCT outcomes be improved by BCRi/BCL2a?
Given the pronounced capacity of BCRi/BCL2a to clear bulky nodes by redistributing CLL cells to the circulation, it is tempting to speculate that these agents could be particularly useful in optimizing remission status prior to HSCT. However, responses achieved with BCRi in HR-CLL can be unsteady upon treatment discontinuation: some patients present with tumor flare.54 Because of this, BCRi-mediated responses may be not durable enough to allow the GVL activity to become effective, an issue that deserves investigation. Moreover, a note of caution is raised by preclinical observations suggesting that ibrutinib can promote a Th1-skewed T-cell response pattern by inhibiting interleukin-2–inducible kinase, thereby potentially increasing the GVHD risk.55 Thus, it is uncertain whether HSCT results in CLL could be improved by the novel drugs by increasing the quality of the response at transplant and/or by facilitating the immunotherapeutic disease control if administered posttransplant.
What we do know about novel drugs in HR-CLL
Novel drugs produce high response rates and prolong PFS in HR-CLL.
The reported response rates in patients with relapsed/refractory (R/R) CLL (including HR-CLL as defined by the presence of del[17p]/TP53 mutations) treated with BCRi/BCL2a are uniformly good. The overall response rates (ORRs), according to the International Workshop on Chronic Lymphocytic Leukemia criteria10 for ibrutinib, idelalisib, and ABT-199, are 48% to 71%, 39%, and 85% if used as monotherapy in R/R patients (Table 3); if partial remissions with persistent lymphocytosis are considered, the response rate to ibrutinib approaches 100%. The ORR might be further increased by combining new agents with chemotherapy and/or anti-CD20 antibodies56,57 (Table 3). A first randomized trial of 220 patients with relapsed CLL, including 44% with del(17p)/TP53 mutations, compared idelalisib plus rituximab with placebo plus rituximab. The ORR rates (81% vs 13%) and 6-month progression-free survival (PFS) (93% vs 46%) strongly favored the idelalisib-rituximab arm.58 Similarly, ibrutinib monotherapy resulted in significantly better ORR, PFS, and OS when randomized against ofatumumab in a prospective trial enrolling 391 patients with R/R CLL.59 Finally, investigators from The Ohio State University reported a retrospective study of 174 patients with CLL who harbored del(17p). After a median follow-up of 12 months, 1-year PFS and OS in the 27 patients treated with ibrutinib were 77% and 81%, significantly superior to that of the 58 and 89 patients treated with cyclin-dependent kinase inhibitors or conventional regimens.34 Altogether, the effectiveness of the new agents seems to result in a longer disease control of R/R CLL—even in the presence of high-risk criteria—than with any other currently available treatment with the possible exception of HSCT.
Complete remissions are uncommon, and curative potential is unlikely.
Despite the excellent ORR, complete remissions are seen in only a minority of patients treated with BCRi/BCL2a (Table 3). Ibrutinib resistance could be attributed to newly developing mutations in the Bruton’s tyrosine kinase gene (BTK) or genes downstream of BTK, but also to clonal evolution of driver genetic alterations independent of the BCR pathway.66,67 A continuous decline of the PFS curve in R/R patients has been reported for the idelalisib phase 1/2 monotherapy trial, with a median PFS of 16 months14 and preliminary data suggesting a similar pattern for ABT-199.62 Of note, the emergence of resistance seems to be more likely in those patients who harbor poor prognostic genetic features. The implication from these observations is that a substantial proportion of CLL clonogenic cells escape the antitumor effect of BCRi/BCL2a. In summary, current evidence does not indicate that BCRi/BCL2a per se might have curative potential in HR-CLL.
TP53 abnormalities retain poor prognostic impact in R/R patients treated with BCRi.
In the pivotal study of ibrutinib in 85 patients with R/R CLL, 28 patients whose tumor clone harbored del(17p) had a significantly inferior outcome (26-month PFS of 57%) than 29 patients without del(17p) and del(11p) (26-month PFS of 93%).13 The 7 patients with del(17p)/TP53 mutations treated with effective doses on the idelalisib pilot trial had a PFS of 5 months compared with 41 months for 21 patients without these abnormalities14 (Table 3). There is no valid information on the specific effect of del(17p)/TP53 mutations on response duration after ABT-199. In summary, although published results with new treatment agents, particularly BCRi, are the best ever reported in patients with del(17p)/TP53 mutations, it is doubtful that the poor prognosis conveyed by these genetic abnormalities is abrogated by these drugs. Therefore, further studies in large series of patients with long-term follow-up are needed.
Efficacy of BCRi/BCL2a in transformed CLL is limited.
This notion is supported by the observation that a high proportion of early progressions occurring under ibrutinib and also ABT-199 present as Richter’s transformation despite the attempt to exclude patients with suspected CLL transformation from these trials.13,68 This might be explained by closer scrutiny of patients included in trials or by actual drug-related mechanisms. More likely, this pattern is suggestive of an expansion of preexisting transformed or resistant subclones during BCRi/BCL2a exposure because it can be also observed under chemoimmunotherapy.69 These findings are consistent with the limited activity of BCRi/BCL2a in diffuse large cell lymphoma and Hodgkin lymphoma.70
Novel drugs have a favorable safety profile.
Phase 2 trials in CLL and other B-cell malignancies show that the safety profile of BCRi is relatively favorable. The most common adverse effects of ibrutinib, CC-292, and idelalisib are mild or moderate diarrhea, nausea, hypertension, and fatigue. Grade 3 or higher toxicities are infrequent and mainly consist of neutropenia, thrombocytopenia, upper respiratory tract infections, and pneumonia.13,14,57,58,61,71,72 In addition, specific and potentially serious complications of idelalisib consist of liver toxicity and late-onset colitis,14 as highlighted by a boxed warning accompanying the US Food and Drug Administration approval of the drug. Information about toxicity of ABT-199 is very preliminary, but apart from tumor lysis syndrome, which appears to be controllable by appropriate dosing and preventive measures, mild to moderate gastrointestinal complications, and respiratory infections appear to be frequent. The few higher-grade toxicities are largely a result of neutropenia.62
Costs of long-term adherence to novel drugs.
On the basis of a routine dose of 420 mg daily, costs for ibrutinib could be expected to be higher than 80 000 Euro (100 000 USD) per treatment year. This means that the pure drug costs of ibrutinib treatment will exceed those of a sibling HSCT within 1 year, and those of an unrelated donor HSCT within 2 years, even if real-world costs of transplant, including follow-up and treatment of all complications, are considered.73 For other agents, there is no information yet for estimating a likely treatment cost.
What we don’t know about novel drugs in HR-CLL
What is the long-term efficacy?
The long-term efficacy of novel agents in CLL treatment is largely unknown, the follow-up of patients included in trials being quite short. For example, the longest median observation time reported was 36 months in an update of the ibrutinib pivotal trial.74
Are there markers predicting efficacy?
Besides preliminary data suggesting that BCRi might be less effective in del(17p)/TP53-mutated CLL, other markers could predict BCRi efficacy. For example, mutations of BTK or BTK downstream targets such as PLCy2 may preexist or emerge under ibrutinib exposure and indicate imminent resistance.67
What is the long-term toxicity?
Although the short-term toxicity of BCRi/BCL2a is in most cases mild and easily manageable, there is no information about long-term toxicity. This is relevant because of the pleiotropic effect of these agents (microenvironment, T cells, B cells) and also because they should seemingly be given on a permanent basis.
What is the optimum duration of treatment?
Information on the outcome of patients who discontinue BCRi/BCL2a treatment after achieving response is lacking. However, the mechanisms of action of the novel agents and the persistence of residual disease in most responders strongly suggest that these compounds should be continuously administered with all the implications for compliance and treatment costs.16
How can patients who relapse while receiving novel drugs be rescued?
Preliminary data suggest that patients with early progression under ibrutinib often emerge as having Richter’s transformation with a rapidly fatal prognosis, while those becoming resistant beyond the first year tend to progress with untransformed CLL sensitive to salvage therapy.75 However, information is lacking on the specific prognosis of patients with HR-CLL progressing under BCRi/BCL2a.
Conclusions
With the advent of BCRi/BCL2a,16 next-generation B-cell antibodies,76 immunomodulators,77 and possibly additional drug classes in the near future, the current algorithms for CLL management will inevitably change. The traditional HR-CLL criteria that define HSCT indication may no longer be valid in the upcoming new treatment landscape. As of today, however, the stage for proposing a fully revised evidence-based treatment algorithm for patients with HR-CLL is far from being set. Meanwhile, the HSCT option should not be discarded but should be included in the treatment decision process, considering what is known and what is still uncertain regarding different treatment possibilities.
Advising therapy for patients with HR-CLL requires the close interaction of fully informed patients and experts on CLL management who are fully cognizant of the advances in CLL therapy and latest developments in HSCT. Key elements to be discussed with the patient are shown in Table 4.
Recommendations
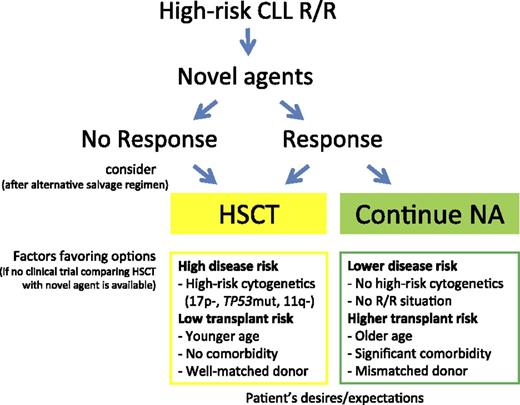
If possible, every patient with HR-CLL in need of treatment should be offered one of the new drugs to induce disease control, ideally within a clinical trial. Once maximum response is achieved, there are 2 options: perform a consolidating HSCT or continue on BCRi/BCL2a until progression, thereby postponing the HSCT option to the next treatment line (Figure 1). In the absence of controlled studies, available evidence does not suggest a general superiority of either of these 2 choices. Survival appears to be comparable with both options during the first 24 months. Importantly, whereas it is well documented that in allografted patients survival decreases only slowly thereafter, the prognosis of patients continuing on BCRi/BCL2a beyond 2 years is largely unknown.
Accordingly, no general recommendation can be given for immediate vs deferred HSCT in patients with HR-CLL who achieve a response to treatment with BCRi/BCL2a. Each decision has to be made after careful discussion of the risks and chances of the 2 options, taking into account the individual’s situation based on a careful clinical and laboratory workup.78 It is important however, that HSCT is not considered a last resort treatment, offered to patients only after all other options have been exhausted. Aspects to be considered include access to new agents, prior treatment, disease risk (R/R situation, genetics), HSCT risk (eg, donor match, frailty, and comorbidity), and the patient’s desires and expectations. Conditions potentially favoring immediate transplantation in HR-CLL are coincidence of R/R HR-CLL with del(17p)/TP53 mutations and/or del(11q), younger age and no significant comorbidity, and availability of a well-matched donor.43 Conversely, factors supporting HSCT deferral are the absence of an R/R situation, R/R situation in the absence of del(17p)/TP53 mutations and del(11q), age >70 years and significant comorbidity, or only a partially matched or mismatched donor available (Figure 1). The same applies to patients with HR-CLL who are referred for transplant evaluation while being in remission upon traditional chemoimmunotherapy without prior exposure to BCRi/BCL2a.
If possible, the 2 options (BCRi/BCL2a vs HSCT) should be compared within clinical trials. If a trial is not available, follow-up and analysis of both transplant and nontransplant strategies on an intention-to-treat basis within the framework of an observational study are strongly recommended. Finally, HSCT should generally be performed only in institutions that have experience and that have an effective accredited quality management system in place.
Future trends
HSCT and new agents may eventually turn out to be complementary rather than competing treatment options. For example, the new B-cell–targeting compounds, including BCRi/BCL2a and next-generation B-cell antibodies, might be used to improve disease control in the early posttransplant phase until GVL activity becomes effective (provided that BCRi/BCL2a do not increase the risk of GVHD). At the same time, continuing posttransplant B-cell depletion could help attenuate chronic GVHD.79,80 The ultimate goal would be to design integrated treatment strategies in which tailored GVL activity acts in concert with targeting agents to achieve durable control of HR-CLL.54,81 Moreover, innovative approaches using cellular therapy, such as B-cell–directed chimeric antigen receptor–engineered T cells may open new pathways for CLL eradication in addition to or instead of HSCT.82,83 Finally, since CLL treatment is advancing so rapidly, it is necessary to continuously factor in new evidence to adapt CLL management algorithms to scientific progress.
There is an Inside Blood Commentary on this article in this issue.
Authorship
Contribution: P.D., J.S., and E.M. designed the concept and wrote the manuscript; and all other authors contributed to further development of the concept, helped write the manuscript, and approved the final version of the manuscript.
Conflict-of-interest disclosure: P.D. is a board member of the European Society for Blood and Marrow Transplantation and the German Working Group on Marrow and Blood Transplantation. He has received consultancy honoraria from Janssen and Gilead. S.S. has received honoraria and research grants from AbbVie, Gilead, Janssen, Pharmacyclics, and Roche. J.G. has served on advisory boards for Roche/Genentech, Pharmacyclics, Janssen, Gilead, AbbVie, Mundipharma, and Celgene. The remaining authors declare no competing financial interests.
Correspondence: Peter Dreger, University of Heidelberg, INF 410, Heidelberg 69120, Germany; e-mail: peter.dreger@med.uni-heidelberg.de.