Key Points
The D′D3 domains of VWF are sufficient to stabilize FVIII in vivo.
The prolongation of VWF D′D3 survival in vivo by Fc fusion elevates FVIII levels in the setting of VWF but not FVIII deficiency.
Abstract
Plasma factor VIII (FVIII) and von Willebrand factor (VWF) circulate together as a complex. We identify VWF fragments sufficient for FVIII stabilization in vivo and show that hepatic expression of the VWF D′D3 domains (S764-P1247), either as a monomer or a dimer, is sufficient to raise FVIII levels in Vwf−/− mice from a baseline of ∼5% to 10%, to ∼50% to 100%. These results demonstrate that a fragment containing only ∼20% of the VWF sequence is sufficient to support FVIII stability in vivo. Expression of the VWF D′D3 fragment fused at its C terminus to the Fc segment of immunoglobulin G1 results in markedly enhanced survival in the circulation (t1/2 > 7 days), concomitant with elevated plasma FVIII levels (>25% at 7 days) in Vwf−/− mice. Although the VWF D′D3-Fc chimera also exhibits markedly prolonged survival when transfused into FVIII-deficient mice, the cotransfused FVIII is rapidly cleared. Kinetic binding studies show that VWF propeptide processing of VWF D′D3 fragments is required for optimal FVIII affinity. The reduced affinity of VWF D′D3 and VWF D′D3-Fc for FVIII suggests that the shortened FVIII survival in FVIII-deficient mice transfused with FVIII and VWF D′D3/D′D3-Fc is due to ineffective competition of these fragments with endogenous VWF for FVIII binding.
Introduction
Loss of procoagulant factor VIII (FVIII) function results in pathologic bleeding. Noncovalent binding to von Willebrand factor (VWF) protects FVIII from degradation and rapid clearance from plasma.1 FVIII levels in patients with von Willebrand disease (VWD) type 1 or type 3 are generally reduced concomitant with decreased levels of plasma VWF. Patients with VWF mutations interfering with VWF:FVIII binding (VWD type 2N) exhibit normal plasma VWF but reduced plasma FVIII levels.2 Infusion of plasma fractions from hemophilia A (HA) patients (containing VWF but no FVIII) into severe VWD patients restores plasma FVIII levels, demonstrating that exogenous VWF stabilizes endogenous FVIII.3 Similarly, treatment of HA patients with recombinant FVIII free of VWF is successful because of the stabilization of exogenous FVIII by endogenous VWF.4 In contrast, correction of low FVIII levels in patients with type 2N or type 3 VWD requires infusion of VWF-containing plasma concentrates.5
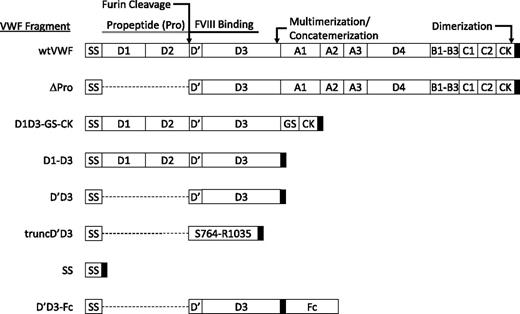
Secreted from endothelial cells as a multimeric glycoprotein, VWF is composed of a series of repeated domains (Figure 1). In the endoplasmic reticulum, proVWF subunits dimerize via intermolecular disulfide bonds at the C-terminal CK domain.6 In the trans-Golgi and post-Golgi compartments, the VWF propeptide catalyzes the formation of VWF multimers via disulfide bonds near the VWF N terminus (C1099-C1099 and C1142-C1142) and is cleaved from proVWF by furin.7-9 The resulting mature VWF multimers condense into long, helical structures that characterize the shape of Weibel–Palade bodies and are released into the circulation as long, linear polymers with multiple concatemerized subunits. Each VWF multimer is composed of 2 to >60 subunits10 (with each monomer containing a single FVIII-binding site). Although plasma FVIII binds to all sizes of multimeric VWF with similar affinities (estimated KD = ∼0.2-0.3 nM), the FVIII:VWF monomer stoichiometry is only ∼1:50.11
Domain structure of VWF and VWF fragments. Each VWF subunit is composed of multiple domains. The expected valency for the mature form of each VWF fragment is described in the “Methods” section. SS denotes the VWF signal sequence. The D1 and D2 domains (A23-R763) compose the VWF propeptide. Furin cleaves between R763 and S764. The amino acid sequence for a minimal, proteolytic VWF fragment previously found to bind FVIII (S764-R1035)14 lies in the D′ and D3 domains. The approximate locations of disulfide bonds that result in dimerization or multimerization/concatemerization of VWF subunits are indicated. VWF domains contained within each subcloned VWF fragment are depicted as white boxes (deletion is indicated by dashed line). The position of the E and FLAG tags is indicated by the black box. GS denotes glycine-serine–rich linker. The Fc residues (G242-K463) for D′D3-Fc are numbered according to the coding sequence of the mRNA for Mus musculus immunoglobulin heavy chain complex (GenBank# BC003435).
Domain structure of VWF and VWF fragments. Each VWF subunit is composed of multiple domains. The expected valency for the mature form of each VWF fragment is described in the “Methods” section. SS denotes the VWF signal sequence. The D1 and D2 domains (A23-R763) compose the VWF propeptide. Furin cleaves between R763 and S764. The amino acid sequence for a minimal, proteolytic VWF fragment previously found to bind FVIII (S764-R1035)14 lies in the D′ and D3 domains. The approximate locations of disulfide bonds that result in dimerization or multimerization/concatemerization of VWF subunits are indicated. VWF domains contained within each subcloned VWF fragment are depicted as white boxes (deletion is indicated by dashed line). The position of the E and FLAG tags is indicated by the black box. GS denotes glycine-serine–rich linker. The Fc residues (G242-K463) for D′D3-Fc are numbered according to the coding sequence of the mRNA for Mus musculus immunoglobulin heavy chain complex (GenBank# BC003435).
FVIII is a heterodimer composed of a heavy chain (domains A1-a1-A2-a2-B) and a light chain (a3-A3-C1-C2) noncovalently linked through divalent cations, which binds to VWF via the FVIII light chain. The acidic a3, C1, and C2 domains of the FVIII light chain are thought to form the noncontiguous interface with VWF.12,13 The FVIII binding region within VWF has been localized to a proteolytic VWF fragment that spans residues S764-R1035, which compose the N terminus of the D′D3 domains and excludes the cysteines that coordinate multimerization.8,14 Removal of the N-terminal S764-L1039 segment by granzyme M cleavage disrupts FVIII binding to multimeric VWF.15 Multiple biochemical methods and the location of VWD type 2N mutations implicate the VWF D′ domain (S764-A865) as the site for FVIII docking.16-20 However, a S764-R1035 proteolytic fragment binds FVIII with markedly reduced affinity (KD = 48.5 nM) compared with longer VWF fragments.21,22
Several approaches have attempted to engineer FVIII with an extended plasma half-life (t1/2).23-26 Recombinant human B-domain deleted FVIII infused into a C57BL/6 mouse has a similar t1/2 (4.3 hours) to that of endogenous murine VWF (∼4.5 hours).25,27,28 Selective conjugation of polyethyleneglycol (PEG)24,26 or fusion of FVIII to the Fc domain of immunoglobulin G (IgG)25 increases FVIII plasma t1/2 by ∼1.5- to 2-fold in mice24-26 and humans.29 Here, we report the effect of a set of VWF fragments on FVIII binding in vitro and survival in vivo and demonstrate a markedly extended plasma t1/2 for an Fc fusion to a 484-amino acid N-terminal VWF fragment, with a concomitant increase in plasma FVIII levels in VWF-deficient but not HA mice.
Materials and methods
VWF expression constructs
The hepatic-specific expression vector, pLIVE (Mirus Bio), was modified to express fragments of murine Vwf complementary DNA (cDNA) from strain A/J30 with C-terminally fused, tandem peptide tags (E and FLAG, herein referred as pLIVE-EF); a glycine hinge (GGRGG) separated the expressed VWF fragments from the tags. The set of VWF fragments cloned into pLIVE-EF are shown in Figure 1. SS contains only the 22-amino acid VWF signal sequence (M1-C22); D′D3 directs expression of the VWF signal peptide fused to a monomeric fragment encompassing the VWF D′ and D3 domains (S764-P1247); truncD′D3 encodes the signal peptide fused to VWF amino acids S764-R1035; D1-D3 includes the signal peptide and VWF propeptide through the D3 domain (M1-P1247), directing expression of a dimeric fragment; and D1D3-GS-CK adds the C-terminal CK domain to D1-D3 (M1-P1247, G2713-K2813), separated by a glycine-serine rich linker [GGRG(G3S)3(EG3S)6(G3S)1GSGGRG]. Expression of D1D3-GS-CK should result in multimers of VWF lacking the A1-C2 domains. ∆Pro deletes the propeptide and fuses the VWF signal peptide to the mature VWF sequence (M1-C22, S764-K2813), which should result in expression of dimers of mature VWF. wtVWF includes the full VWF cDNA sequence (M1-K2813) and should direct expression of wild type (wt) VWF multimers. Finally, C-terminal fusion of the Fc portion of IgG1 (contiguously spanning the IgG1 hinge region to the CH3 domain) to the D′D3 sequence described above generated D′D3-Fc. For expression of recombinant proteins for bio-layer interferometry binding studies, truncD′D3, D′D3, D1-D3, and D′D3-Fc were subcloned into pcDNA3.1-V5-HisA (Invitrogen). The assembly of all VWF expression constructs is detailed in the supplemental Methods on the Blood Web site.
In vitro protein expression and purification
HEK293T or HepG2 cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Invitrogen) containing 10% fetal bovine serum and transiently transfected at a DNA-polyethylenimine (branched polyethylenimine, Sigma) ratio of 1 mg DNA to 4 mL polyethylenimine (1 mg/mL) with Opti-MEM (Invitrogen). Conditioned media from HEK293T cells were collected 2 to 3 days after transient transfection and sterile-filtered (0.2 μm). The media overlaying HepG2 cells were changed to DMEM (serum-free) 1 day after transfection, and the conditioned media were collected after 2 to 3 days and clarified by sterile filtration (0.2 μm) or centrifugation.
Recombinant VWF fragments were immunoprecipitated from the clarified conditioned media with M2 anti-FLAG agarose beads (Sigma-Aldrich) at 4°C overnight. Beads were recovered by passing the solution through Econo-Pac chromatography columns (Bio-Rad). Beads were then washed with phosphate buffered saline (Invitrogen) containing 0.05% Tween-20 and 100 μM 4-(aminoethyl)benzenesulfonyl fluoride hydrochloride (Sigma-Aldrich), HBS (20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid + 150 mM NaCl, pH = 7.4), or with HBS-T (HBS + 0.001% Tween-20). VWF fragments were eluted with 150 ng/μL 3X FLAG peptide (Sigma-Aldrich), concentrated on a 3- or 10-kDa Amicon Ultra centrifugal filter (Millipore), and further purified by gel filtration through Superose 6 (GE Life Sciences; all VWF fragments except truncD′D3) or Bio-Gel P-100 (Bio-Rad; only for truncD′D3) in a Tricorn 10/300 column (GE Life Sciences). Fractions containing VWF fragments were concentrated on a 3-, 10-, or 30-kDa Amicon Ultra centrifugal filter. For proteins concentrated on a 3-kDa centrifugal filter, Tween-20 was added to 0.001%. Final protein concentrations were measured using the BCA protein assay kit (Thermo-Fisher) or the Coomassie protein assay kit (Thermo-Fisher).
Mouse procedures
Mice were bred and housed at the University of Michigan. All procedures were approved by the University of Michigan's Committee on Use and Care of Animals.
In vivo protein expression (hydrodynamic injection)
Vwf−/− mice (backcrossed ≥10 generations onto C57BL/6J) were injected in a lateral tail vein with 50 μg DNA dissolved in Dulbecco’s phosphate-buffered saline (without Ca2+ or Mg2+, Invitrogen) at a volume equivalent to 10% of the body mass (ie, 0.1 mL/g), similarly to as previously described.31 Blood was collected from the retro-orbital plexus or from the right ventricle into sodium citrate (Sigma; final concentration = 0.4%, wt/vol). Platelet poor plasma (PPP) was prepared by centrifugation at 2000g for 10 minutes at room temperature and stored at −80°C.
Clearance studies
Vwf−/− (C57BL/6J genetic background) or HA (backcrossed ≥20 generations onto 129S1/SvIm) mice were intravenously transfused with pooled PPP (200 μL per recipient mouse) derived from hydrodynamically injected mice. For all clearance studies, these PPP were used as the source of VWF fragments rather than recombinant proteins expressed in vitro because of the high levels of protein expressed in vivo and previously reported differences in half-lives between tissue culture–derived murine VWF32 and plasma-derived murine VWF.27 Citrated blood from recipient mice was collected at indicated time points, and PPP was prepared and stored at −80°C until analyzed. Clearance rates were estimated by single-phase, nonlinear regression in Prism (GraphPad).
FVIII activity
FVIII levels in PPP, thawed at 37°C, were measured using the acid-stopped method of COATEST (Diapharma) according to the manufacturer’s instructions.
VWF ELISA
VWF levels were measured by enzyme-linked immunosorbent assay (ELISA). Maxisorp 96-well, U-shaped bottom plates (Nunc) were coated with 1 μg/mL goat-anti-ECS (goat-anti-FLAG; Abcam or Bethyl Labs) or with 1:500 rabbit-anti-VWF (Dako) in a 50-mM carbonate/bicarbonate buffer (pH = 9.8) at 4°C overnight. Coated plates were washed with Tris-buffered saline with Tween 20 (TBST; 50 mM Tris-HCl, pH = 7.4, + 150 mM NaCl + 0.05% Tween-20) and blocked with 5% bovine serum albumin (BSA, Sigma) in TBST (BSA/TBST) at room temperature. VWF fragments (from PPP) diluted in 5% BSA/TBST were adsorbed onto the coated/blocked plates at room temperature. After washing with TBST, VWF fragments were probed with 0.04 μg/mL goat-anti-E tag-horseradish peroxidase (HRP) (Abcam) or 1:2000 rabbit-anti-VWF-HRP (Dako) diluted in 5% BSA/TBST at room temperature. Wells were washed with TBST and developed with 3,3′,5,5′-tetramethylbenzidine-ELISA (Thermo Scientific). After stopping the reaction with 2 M H2SO4, the absorbance was measured at 450 nm. Recombinant VWF fragments purified from tissue culture were used as ELISA standards.
Western blot
HepG2 conditioned media containing VWF fragments were concentrated on 3-kDa Amicon Ultra centrifugal filters. Equal volumes of concentrated conditioned media (5-10 μL) or PPP (0.5-1 μL) from hydrodynamically injected mice were denatured under reducing or nonreducing conditions. Denatured samples were fractioned by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis in 25 mM Tris + 192 mM glycine + 1% SDS and transferred onto nitrocellulose in 25 mM Tris + 192 mM glycine + 20% methanol. Membranes were blocked in blocking buffer (5% nonfat, powdered milk in TBST), probed with 0.05 μg/mL goat-anti-FLAG-HRP (Abcam) in blocking buffer, and developed with SuperSignal West Pico (Thermo Scientific).
Recombinant wtVWF was analyzed by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described above. Multimer analysis (ie, nonreducing condition) of recombinant wtVWF was performed as described by Ott et al, with modifications.33 Briefly, concentrated conditioned media or PPP (sample volume equal to that of reducing condition) were denatured in 20 μL multimer sample buffer (70 mM Tris, pH = 6.8, + 4 mM EDTA + 9 M urea + 83.22 mM SDS + 0.5 mg/mL bromophenol blue). VWF multimers were resolved through a 1% agarose gel (SeaKem HGT(P), Lonza), transferred onto nitrocellulose (2.5 mM Tris, pH = 8.8, + 19.2 mM glycine + 0.01% SDS + 20% methanol) at 9°C, probed with 0.05 μg/mL goat-anti-FLAG-HRP or with 1.65 μg/mL rabbit-anti-VWF (Dako) in blocking buffer, and developed with SuperSignal West Pico (Thermo Scientific).
Bio-layer interferometry
Recombinant FVIII (Helixate FS, 1000 U) was reconstituted with 0.5 mL sterile water and buffer exchanged into HBS-T supplemented with 2.5 mM CaCl2 and 1% sucrose on a 30-kDa Amicon Ultra centrifugal filter. Protein concentration of the buffer exchanged FVIII was measured using the Coomassie protein assay kit. Aliquots were stored at −80°C.
All binding experiments were performed using the Octet Red (Forte Bio) and kinetics buffer (HBS-T supplemented with 2.5 mM CaCl2, 1 mg/mL BSA, and 1% sucrose) at 22°C. VWF fragments (0.2-2 μg/mL) purified from transiently transfected HEK293T cells were immobilized onto Dip and Read anti-FLAG biosensors (Forte Bio) in the presence of 0.1 μg/mL FLAG peptide (Sigma, F3290). VWF-coated biosensors were washed in kinetics buffer before association and dissociation of various concentrations (1-10 nM) of FVIII. Background wavelength shifts were measured from parallel sensors that were loaded only with FLAG peptide. Background-subtracted wavelength shifts were exported into Prism (GraphPad) and nonlinearly regressed to the “association then dissociation” model to determine kinetic constants.
Statistics
Significance was tested by Student t test. Confidence intervals (CI) were calculated at a 95% confidence level.
Results
In vitro and in vivo expression of VWF fragments produce disulfide bonded structures
Expression and secretion was observed for all studied VWF fragments (except SS) transfected into HepG2 cells or hydrodynamically injected into Vwf−/− mice (supplemental Figure 1). truncD′D3 and D′D3 migrated slower under reducing conditions than under nonreducing conditions (supplemental Figure 1A-B), consistent with the presence of intramolecular disulfide bonds. D1-D3 (supplemental Figure 1C), D1D3-GS-CK (supplemental Figure 1D), ∆Pro (supplemental Figure 1E), and wtVWF (supplemental Figure 1F) migrated at the expected sizes under reducing conditions but slower under nonreducing conditions, consistent with the formation of dimers or multimers via intermolecular disulfide bonds. The full range of multimer sizes were observed for wtVWF (supplemental Figure 1F), though with greater accumulation of smaller multimeric forms compared with plasma VWF, consistent with previous reports of VWF expressed in multiple cell types.31,34
In vivo expression of VWF fragments stabilizes plasma FVIII
Hydrodynamic injection of plasmids resulted in hepatic expression of VWF fragments and secretion into the plasma of Vwf−/− mice (Figure 2A; supplemental Figure 1), consistent with previous reports.31,32 Expected disulfide bridging and multimerization were observed, similar to the recombinant VWF fragments expressed in HepG2 cells (supplemental Figure 1). Peak plasma level for each fragment (∼15-∼70 nM of subunit) was generally observed at 1 to 7 days postinjection and remained detectable throughout the 4-week time course (Figure 2A). The range of VWF fragment concentrations over the period of observation (∼7 to ∼70 nM of subunit) corresponded to ∼47% to ∼470% of VWF levels in C57BL/6J mice (∼15 nM of subunit).
In vivo expression of VWF fragments elevates basal FVIII levels in Vwf−/− mice. Circulating VWF levels (A) and FVIII levels (B) were measured from 6 to 8 hydrodynamically injected Vwf−/− mice at the indicated time points (mean ± standard deviation [SD]). (A) VWF concentrations measured in the PPP were converted to molarity of subunits based on the molecular mass of each VWF fragment calculated from the amino acid sequence (wtVWF = 228 kDa, ∆Pro = 228 kDa, D1D3-GS-CK = 71 kDa, D1-D3 = 56 kDa, D′D3 = 56 kDa, truncD′D3 = 33 kDa). The black line corresponds to the VWF level in pooled PPP from 10 C57BL/6J mice (3.4 μg/mL). Preinjection VWF levels were undetectable by ELISA. Plasma levels were not measured for SS. The width of the error bars likely stemmed from the highly variable response to the hydrodynamic injection and subsequent recovery from this injury. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. d, day; wk, week.
In vivo expression of VWF fragments elevates basal FVIII levels in Vwf−/− mice. Circulating VWF levels (A) and FVIII levels (B) were measured from 6 to 8 hydrodynamically injected Vwf−/− mice at the indicated time points (mean ± standard deviation [SD]). (A) VWF concentrations measured in the PPP were converted to molarity of subunits based on the molecular mass of each VWF fragment calculated from the amino acid sequence (wtVWF = 228 kDa, ∆Pro = 228 kDa, D1D3-GS-CK = 71 kDa, D1-D3 = 56 kDa, D′D3 = 56 kDa, truncD′D3 = 33 kDa). The black line corresponds to the VWF level in pooled PPP from 10 C57BL/6J mice (3.4 μg/mL). Preinjection VWF levels were undetectable by ELISA. Plasma levels were not measured for SS. The width of the error bars likely stemmed from the highly variable response to the hydrodynamic injection and subsequent recovery from this injury. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. d, day; wk, week.
Baseline FVIII levels in Vwf−/− mice (9% to 16%) were unchanged following hydrodynamic injection of SS, whereas wtVWF restored FVIII to ∼100% to 200% of the level in C57BL/6J (P < .01 vs preinjection), consistent with previous reports.31,35 In the latter experiment, FVIII levels closely paralleled VWF antigen levels over the period of observation (Pearson’s correlation coefficient = 0.80, 95% CI = 0.70-0.90), as similarly observed in humans.36 Expression of ∆Pro, D1D3-GS-CK, D1-D3, and D′D3 all resulted in markedly increased FVIII levels (P < .01 vs preinjection), ranging from 50% to 120% from day 1 through 4 weeks postinjection (Figure 2B). truncD′D3 produced a minimal, transient elevation in FVIII levels.
D′D3 VWF fragments exhibit accelerated clearance compared with wtVWF
The stable VWF fragment levels over 4 weeks (Figure 2A) likely reflect an equilibrium between continuous synthesis/secretion from plasmid-transfected hepatocytes and clearance from the circulation. To directly measure VWF fragment clearance, naive Vwf−/− mice were transfused with PPP obtained from Vwf−/− mice that had been previously hydrodynamically injected with each VWF fragment–expressing plasmid. All transfused VWF fragments cleared from the circulation of recipient Vwf−/− mice within 24 hours (Figure 3). wtVWF demonstrated a t1/2 of 3.7 ± 1.7 hours, consistent with previous reports.27,37 The t1/2 observed for ∆Pro (2.4 ± 0.4 hours) was indistinguishable from wtVWF (P > .11). However, all 3 D′D3 fragments exhibited reduced t1/2 (D′D3 = 1.5 ± 0.3 hours, D1-D3 = 1.0 ± 0.2 hours, D1D3-GS-CK = 0.8 ± 0.2 hours; P < .05 vs wtVWF), likely because of stabilizing effects of the other VWF domains on protein folding and plasma clearance.
Transfused VWF fragments exhibit t1/2 of ∼1 to ∼4 hours (hrs). PPP isolated from hydrodynamically injected Vwf−/− mice (n = 5-11 mice per VWF fragment) by cardiac puncture was pooled and transfused into 4 to 9 naive Vwf−/− mice. Residual VWF levels (mean ± SD) in recipient mice were normalized to the 5-minute time point (set as 100% [%5min]). Measurements were nonlinearly regressed to predict the clearance of each FVIII-stabilizing VWF fragment (curves).
Transfused VWF fragments exhibit t1/2 of ∼1 to ∼4 hours (hrs). PPP isolated from hydrodynamically injected Vwf−/− mice (n = 5-11 mice per VWF fragment) by cardiac puncture was pooled and transfused into 4 to 9 naive Vwf−/− mice. Residual VWF levels (mean ± SD) in recipient mice were normalized to the 5-minute time point (set as 100% [%5min]). Measurements were nonlinearly regressed to predict the clearance of each FVIII-stabilizing VWF fragment (curves).
Fc fusion markedly prolongs D′D3 survival and FVIII elevation in Vwf−/− mice
Expression of D′D3 fused to the Fc portion of IgG1 (D′D3-Fc) in HepG2 cells or by hydrodynamic injection into Vwf−/− mice resulted in secretion of a disulfide-bonded dimer of the expected size (supplemental Figure 2). In contrast to the other VWF fragments and wtVWF, D′D3-Fc exhibited a markedly extended circulatory t1/2 (>7 days, Figure 4A) compared with wtVWF or D′D3. Transfused Vwf−/− mice retained ∼70% of D′D3-Fc in the circulation at 1 week, whereas wtVWF and D′D3 were no longer detectable after 48 hours. The prolonged plasma survival of D′D3-Fc resulted in a corresponding prolongation in the elevation of FVIII levels (Figure 4B). FVIII levels in mice receiving wtVWF or D′D3 peaked at 2 to 10 hours posttransfusion, lagging behind VWF levels as observed in VWD patients receiving VWF concentrates,38 and returned to baseline by 48 hours. D′D3-Fc markedly elevated plasma FVIII levels, which were sustained through the entire period of observation (Figure 4B).
D′D3-Fc is retained in the circulation and elevates FVIII plasma levels for >7 days in Vwf−/− mice. VWF fragment levels (normalized to the value at 5 minutes as 100% [%5min], A) and FVIII levels (B) were followed in Vwf−/− mice (n = 4-5) transfused with 200 μL pooled PPP derived from hydrodynamically injected Vwf−/− mice (n = 5-7). Donor PPP composition (VWF in nM of subunit, FVIII in %C57BL/6J): D′D3-Fc (9.5 nM, 41.8%), D′D3 (4.9 nM, 67.2%), and wtVWF (49.7 nM, 108.6%). (A) VWF levels for wtVWF and D′D3 shown here are from a subset of mice included in Figure 3 whose FVIII levels were also measured. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. The mean pretransfusion FVIII levels from all treated Vwf−/− mice (n = 14) are indicated by the black line (8.5%). Data (mean ± SD) were nonlinearly regressed (curves).
D′D3-Fc is retained in the circulation and elevates FVIII plasma levels for >7 days in Vwf−/− mice. VWF fragment levels (normalized to the value at 5 minutes as 100% [%5min], A) and FVIII levels (B) were followed in Vwf−/− mice (n = 4-5) transfused with 200 μL pooled PPP derived from hydrodynamically injected Vwf−/− mice (n = 5-7). Donor PPP composition (VWF in nM of subunit, FVIII in %C57BL/6J): D′D3-Fc (9.5 nM, 41.8%), D′D3 (4.9 nM, 67.2%), and wtVWF (49.7 nM, 108.6%). (A) VWF levels for wtVWF and D′D3 shown here are from a subset of mice included in Figure 3 whose FVIII levels were also measured. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. The mean pretransfusion FVIII levels from all treated Vwf−/− mice (n = 14) are indicated by the black line (8.5%). Data (mean ± SD) were nonlinearly regressed (curves).
Prolonged survival of D′D3-Fc does not sustain plasma FVIII in transfused HA mice
To test the stability of the D′D3-Fc/FVIII complex in the presence of endogenous VWF but absence of endogenous FVIII, naive HA mice were transfused with PPP from hydrodynamically injected Vwf−/− mice, which express hepatically derived D′D3-Fc and endogenous FVIII. As in recipient Vwf−/− mice, D′D3-Fc levels remained elevated at >∼60% of the initial posttransfusion level in recipient HA mice for >1 week (P > .41, Figure 5A). The t1/2 of D′D3 (2.1 ± 0.6 hours) in HA mice was similar to that observed in Vwf−/− mice (P > .16). In contrast, wtVWF demonstrated a significantly longer t1/2 in HA mice (12.4 ± 1.3 hours) than in Vwf−/− mice (3.7 ± 1.7 hours, P < .0001), likely because of genetic strain differences between 129S1/SvIm and C57BL/6J mice (see the “Discussion” section).
Fc fusion prolongs D′D3 but not FVIII survival in HA mice. VWF fragment levels (normalized to the value at 5 minutes as 100% [%5min], A) and FVIII levels (B) were followed in HA mice (n = 4-9) transfused with 200 μL pooled PPP derived from hydrodynamically injected Vwf−/− mice (n = 5-6). Donor PPP composition (FVIII in %C57BL/6J, VWF in nM of subunit): wtVWF (122.6%, 15.5 nM), D′D3 (71.0%, 3.4 nM), and D′D3-Fc (90.0%, 3.2 nM). Data (mean ± SD) were nonlinearly regressed (curves) as a single-phase exponential decay. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. The FVIII levels in mice transfused with D′D3-Fc were not regressed because of insufficient time points; a line is drawn through the data points for visual assistance.
Fc fusion prolongs D′D3 but not FVIII survival in HA mice. VWF fragment levels (normalized to the value at 5 minutes as 100% [%5min], A) and FVIII levels (B) were followed in HA mice (n = 4-9) transfused with 200 μL pooled PPP derived from hydrodynamically injected Vwf−/− mice (n = 5-6). Donor PPP composition (FVIII in %C57BL/6J, VWF in nM of subunit): wtVWF (122.6%, 15.5 nM), D′D3 (71.0%, 3.4 nM), and D′D3-Fc (90.0%, 3.2 nM). Data (mean ± SD) were nonlinearly regressed (curves) as a single-phase exponential decay. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. The FVIII levels in mice transfused with D′D3-Fc were not regressed because of insufficient time points; a line is drawn through the data points for visual assistance.
In contrast to the results in Vwf−/− mice, transfusion of PPP containing D′D3 or D′D3-Fc did not prolong cotransfused FVIII survival in HA mice (Figure 5B). FVIII was no longer detectable in HA mice at 24 hours posttransfusion of either D′D3 fragment-containing PPP. The donated FVIII (t1/2 = 5.7 ± 0.6 hours) cleared more slowly than the D′D3 antigen (t1/2 = 2.1 ± 0.6 hours, P < .0005). FVIII cotransfused with wtVWF survived the longest in HA mice (t1/2 = 9.0 ± 0.3 hours, P < .001 vs D′D3). Posttransfusion FVIII levels in these mice closely correlated with cotransfused wtVWF levels (Pearson’s correlation coefficient = 0.93, 95% CI = 0.91 to 0.94).
Absence of propeptide processing weakens the affinity for FVIII
Binding constants were measured between FVIII and VWF fragments (D1-D3, D′D3, D′D3-Fc, and truncD′D3; supplemental Figure 3). D1-D3 showed a dissociation constant (Table 1) consistent with previously reported dissociation constants for multimeric VWF.39 D′D3, D′D3-Fc, and truncD′D3 all bound FVIII with a reduced affinity relative to D1-D3 (Table 1) primarily because of faster rates of FVIII dissociation (ie, ∼2- to ∼7-fold greater koff; Table 1).
Discussion
Survival of circulating FVIII relies on its interaction with plasma VWF. The t1/2 of recombinant FVIII or recombinant human B-domain deleted FVIII infused into mice is significantly shorter in the absence than presence of VWF (18 minutes vs 5.9-7.6 hours, respectively).24-26 We confirm this relationship and demonstrate that a minimal, N-terminal VWF fragment comprising D′D3 domains (S764-P1247) is sufficient to stabilize endogenous FVIII in Vwf−/− mice. Extending the circulatory lifetime of D′D3 by Fc fusion markedly prolongs endogenous FVIII survival in Vwf−/− mice but is insufficient to extend the FVIII t1/2 in HA mice.
Plasma FVIII levels closely correlate with plasma VWF levels36 because of the dependence of FVIII on its tight interaction with VWF for stability in the circulation.1 The VWF level we observed in C57BL/6J mice (3.4 μg/mL, Figure 2A) is ∼1/3 of the average VWF level in healthy humans, consistent with a previous report.40 Whereas VWF levels in healthy humans range ∼3-fold (from ∼5 to ∼15 μg/mL),41 VWF levels among inbred mouse strains vary even more widely (>10-fold).30 This latter variation is due to differences in VWF synthesis/secretion and clearance among mouse strains.28,30 Genetic differences between the C57BL/6J and 129S1/SvImJ strains likely explain the more rapid clearance of wtVWF we observed in Vwf−/− mice (C57BL/6J background) compared with similarly transfused HA mice (129S1/SvImJ background). Because the same source of transfused VWF was used in both experiments, these data suggest that the ∼2.5-fold difference in steady-state VWF levels between these strains42 is primarily from an underlying clearance mechanism, rather than a difference intrinsic to the VWF protein itself. These data would also explain the longer t1/2 of transfused FVIII we observed in HA mice (129S1/SvImJ genetic background) compared with previous reports using HA mice on a C57BL/6 genetic background.24-26
The stoichiometry of VWF:FVIII is ∼50:1 in human plasma.43 Assuming a human plasma FVIII concentration of ∼1 nM43 and that FVIII levels in C57BL/6J mice are ∼0.26-fold of human levels,40 we estimate a similar stoichiometry (∼57:1) for the VWF/FVIII complex in C57BL/6J mice. Similar VWF/FVIII stoichiometry (∼52:1, 95% CI = 32:1-72:1) is observed for Vwf−/− mice hydrodynamically injected with the wtVWF plasmid DNA, demonstrating equivalent FVIII interaction for VWF expressed in hepatocytes compared with endogenous endothelial-derived VWF.
Mature VWF adopts a conformation for the N-terminal FVIII binding domain that supports optimal FVIII binding. VWF mutations that prevent propeptide cleavage result in impaired FVIII binding,44-46 whereas VWF expressed without the propeptide exhibits a 6-fold lower affinity for FVIII in vitro than full-length, multimeric VWF.47 Together, these reports suggest that the VWF propeptide mediates optimal folding of the D′D3 domains for FVIII binding. Our results demonstrate that propeptide-mediated folding is not required for FVIII stabilization in vivo, and that expressing the N-terminal VWF D′D3 domains alone is sufficient to support FVIII stabilization in plasma. However, the failure of truncD′D3 to elevate levels of endogenous plasma FVIII, despite measurable FVIII binding in vitro (Table 1), suggests that residues C-terminal to R1035 are required for FVIII stabilization, potentially through an effect on protein folding. Of note, in vivo expression of D1D3-GS-CK, which replaces the VWF A1-C2 domains with a glycine-serine-rich linker, elevates plasma FVIII levels less well than wtVWF (Figure 2B), suggesting that VWF segments between the D3 and CK domains contribute to FVIII stabilization, likely through an effect of protein folding/conformation or other posttranslational modification(s).
Fc fusion to therapeutic proteins prolongs circulatory lifetime by redirecting the fusion product to the neonatal Fc receptor (FcRn) mediated recycling pathway.48 This latter mechanism mediates the exceptionally long t1/2 of plasma immunoglobulins. After internalization into endothelial or hematopoietic cells, endosomal acidification promotes strong binding between the Fc of IgG and its receptor, FcRn, which diverts IgG from lysosomal catabolism to cellular exocytosis.48,49 This strategy has been exploited to engineer recombinant therapeutic proteins with markedly prolonged t1/2.25,50-52 Though Fc fusion extends the t1/2 for clotting factor IX by ∼3- to 4-fold in humans and mice with hemophilia B,50,53 the t1/2 for Fc-fused FVIII is only extended ∼1.5- to 2-fold in mice, dogs, and humans with HA.25,29 This limited effect of Fc fusion on FVIII is likely related to the dependence of FVIII on VWF, as demonstrated by the marked elevation of plasma FVIII in Vwf−/− mice transfused with D′D3-Fc.
The interaction between FVIII and D’D3-Fc is dynamic. In contrast to the persistent elevation of FVIII in Vwf−/− mice transfused with D′D3-Fc containing plasma, FVIII is cleared more quickly in similarly transfused HA mice, despite persistent high levels of VWF D′D3-Fc. This difference is likely explained by the continued production of endogenous FVIII in Vwf−/− mice and VWF in the HA mice. In Vwf−/− mice transfused with D′D3-Fc, FVIII dissociated from this fragment would be replaced by newly synthesized, endogenous FVIII. Conversely, HA mice incapable of FVIII production continuously synthesize endogenous VWF that competes with D′D3-Fc for cotransfused FVIII. The more rapid clearance of D′D3 in HA mice than the cotransfused FVIII presumably results from this competition. Together with our dissociation constant estimates, these data confirm that VWF propeptide processing of the D′D3 domains (ie, D1-D3 and multimeric VWF) results in structural features that strengthen VWF’s affinity for FVIII, consistent with a previous report.47 Significantly less FVIII remains in the circulation at 4 hours posttransfusion when cotransfused with D′D3-Fc than with D′D3 (23.8 ± 9.3% vs 54.4 ± 5.7%, normalized to 5 minutes; P < .005), suggesting that the fraction of FVIII bound to D′D3-Fc may be lost during cellular uptake and FcRn-mediated recycling. The acidic pH within the endosome (∼6.0) that promotes Fc binding to FcRn could contribute to disruption of the VWF-FVIII interaction.48,54,55
Treatment of patients with type 2N or 3 VWD with FVIII-containing VWF concentrates typically produces elevated levels of VWF and FVIII for ∼24 hours, limited by the t1/2 of VWF (∼12-20 hours).2 Prolonging the circulatory lifetime of VWF or a FVIII-binding VWF fragment (eg, D′D3-Fc) represents a potential approach to more prolonged elevation of FVIII in these patients, requiring less frequent infusions for prophylaxis.
FVIII prophylaxis is rapidly becoming the standard of care for adults as well as children with HA.4 Because of the short t1/2,4 currently available products are typically infused intravenously ∼3 times per week for effective prophylaxis. Thus, there has been considerable interest in developing longer-lived FVIII products that could be administered less frequently. Though modified FVIII (Fc-fused and PEG-conjugated) modestly improve t1/2 and could reduce infusion from 3 to 2 times per week,24,26,29 further extension of t1/2 would be of great clinical value. D′D3 or a similar VWF Fc fusion could prove effective in this setting, though strategies to engineer tighter FVIII binding may be required to offset FVIII dissociation and/or competition with endogenous VWF.
There is an Inside Blood Commentary on this article in this issue.
Presented in abstract form at the 54th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 8, 2012.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Qianyi Ma and Dr Jun Li for statistical assistance and James Delproposto for assistance with bio-layer interferometry.
This work was supported by the Goerlich Foundation, the National Hemophilia Foundation (Judith Graham Pool Postdoctoral Fellowship), the National Institutes of Health (grant HL039693), and the Howard Hughes Medical Institute.
Authorship
Contribution: A.Y., S.W.P., and D.G. designed the experiments; A.Y. and S.G. performed experiments; A.Y., R.D.G., B.M.M., and K.M.C. provided vital reagents; A.Y. collected data; A.Y., C.A.K., S.W.P., and D.G. analyzed and interpreted data; and A.Y. and D.G. wrote the manuscript.
Conflict-of-interest disclosure: D.G. receives royalty income from Boston Children’s Hospital related to the production of recombinant VWF. The other authors declare no competing financial interests.
Correspondence: David Ginsburg: University of Michigan, Life Sciences Institute, 210 Washtenaw Ave, Ann Arbor, MI 48109; e-mail: ginsburg@umich.edu.

![Figure 2. In vivo expression of VWF fragments elevates basal FVIII levels in Vwf−/− mice. Circulating VWF levels (A) and FVIII levels (B) were measured from 6 to 8 hydrodynamically injected Vwf−/− mice at the indicated time points (mean ± standard deviation [SD]). (A) VWF concentrations measured in the PPP were converted to molarity of subunits based on the molecular mass of each VWF fragment calculated from the amino acid sequence (wtVWF = 228 kDa, ∆Pro = 228 kDa, D1D3-GS-CK = 71 kDa, D1-D3 = 56 kDa, D′D3 = 56 kDa, truncD′D3 = 33 kDa). The black line corresponds to the VWF level in pooled PPP from 10 C57BL/6J mice (3.4 μg/mL). Preinjection VWF levels were undetectable by ELISA. Plasma levels were not measured for SS. The width of the error bars likely stemmed from the highly variable response to the hydrodynamic injection and subsequent recovery from this injury. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. d, day; wk, week.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/3/10.1182_blood-2013-11-540534/4/m_445f2.jpeg?Expires=1769262415&Signature=f8wXahUw3Aj4casgVjHg1S0PLfIGGivN58bxa-qekrGRxBWJR2r3--r5ca1DlpHUq3LjO~a8cCskODgPzxZ2ORtHp50H6O8kaeR0sDU-Kr57NtOK0rwzNppOm9atH8VknVteJOPdASLgEeyHssuspRwZJY3idh-PZtOuuCDLo4KHu3zWeFL-WF~XFLwyc-rtEBrzGd9EG0Ptq9QepPR0SSXtv~-hPOAVEqwpy~5DoCzti9kmsWkREN58qOvPdGCyuRovFRW8J9E1DXkXReYnjJAQoatI5GmQb9rI8PZ1AT0XCiFH2IHXNoKMHBP0WtHA9vl4E5etZJYuLaqv4dZX1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Transfused VWF fragments exhibit t1/2 of ∼1 to ∼4 hours (hrs). PPP isolated from hydrodynamically injected Vwf−/− mice (n = 5-11 mice per VWF fragment) by cardiac puncture was pooled and transfused into 4 to 9 naive Vwf−/− mice. Residual VWF levels (mean ± SD) in recipient mice were normalized to the 5-minute time point (set as 100% [%5min]). Measurements were nonlinearly regressed to predict the clearance of each FVIII-stabilizing VWF fragment (curves).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/3/10.1182_blood-2013-11-540534/4/m_445f3.jpeg?Expires=1769262415&Signature=mRVJ9PLF6WWqlTAXlbWRKj1C~AZNcxBxblQ8MZeClDZXy2LeE54Kge7KpBggwJLc~Xjfl0mooz9kxKxTVw7vn~ppRmb8q5vsIff3AObwz4dPQ0RrcjNZjsyIYxw2-8paO7JvvJZzxXbMg50GLO~jrZEExe9sl8vEs4b4bn8I72eeLmYGJZ8xIzzX0XgW2EtyWv4KbRLJJeJrk~SyaxEmnnT8PzvUwB13WJNCBMxn69Y4f~DLBq2Z5NAQboEo3vQzwiTnazsIkxYNT72DnzRAy28dNVaSKjmzBb3SwPqR7-TjJTw3q5H-Sj7OIy9uSv6r~4PYfDsIYh-nYjJpPYlh~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. D′D3-Fc is retained in the circulation and elevates FVIII plasma levels for >7 days in Vwf−/− mice. VWF fragment levels (normalized to the value at 5 minutes as 100% [%5min], A) and FVIII levels (B) were followed in Vwf−/− mice (n = 4-5) transfused with 200 μL pooled PPP derived from hydrodynamically injected Vwf−/− mice (n = 5-7). Donor PPP composition (VWF in nM of subunit, FVIII in %C57BL/6J): D′D3-Fc (9.5 nM, 41.8%), D′D3 (4.9 nM, 67.2%), and wtVWF (49.7 nM, 108.6%). (A) VWF levels for wtVWF and D′D3 shown here are from a subset of mice included in Figure 3 whose FVIII levels were also measured. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. The mean pretransfusion FVIII levels from all treated Vwf−/− mice (n = 14) are indicated by the black line (8.5%). Data (mean ± SD) were nonlinearly regressed (curves).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/3/10.1182_blood-2013-11-540534/4/m_445f4.jpeg?Expires=1769262415&Signature=GqP0RaVSY~VXdc5PChl9BWaA09FUbfiEmWGdHE0GpXPOJMw1pvE0jFoqmzEBoQET4aWOIXe9D-KYz17LptFdUJFOodJU8xNBEh3KyUFT6~VbhCagOOzcT6HWFa8KaTbYB6L4jBOYsS9SMBYJY0EgqMuHabG7on3sP0--epOLuxxv6kJ5TphzgkT1Sra-0j5uviNWHAq6jfU~K3wELq1uu4cUEy8ksFe0ZA4CPS1ve31lerXW4otiW53~a81vqoRc2E80eXPNrMPD4uOXr53hZUkvd0ySxtuXAS00yicDhaFZ3u7dhXmelriPx1Ah49O76RGF6JQxYujJCi-SyELIag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Fc fusion prolongs D′D3 but not FVIII survival in HA mice. VWF fragment levels (normalized to the value at 5 minutes as 100% [%5min], A) and FVIII levels (B) were followed in HA mice (n = 4-9) transfused with 200 μL pooled PPP derived from hydrodynamically injected Vwf−/− mice (n = 5-6). Donor PPP composition (FVIII in %C57BL/6J, VWF in nM of subunit): wtVWF (122.6%, 15.5 nM), D′D3 (71.0%, 3.4 nM), and D′D3-Fc (90.0%, 3.2 nM). Data (mean ± SD) were nonlinearly regressed (curves) as a single-phase exponential decay. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. The FVIII levels in mice transfused with D′D3-Fc were not regressed because of insufficient time points; a line is drawn through the data points for visual assistance.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/3/10.1182_blood-2013-11-540534/4/m_445f5.jpeg?Expires=1769262415&Signature=yY2EUbR2ExumLDjpv9jaaOP6toxmFXtcdtiCPTHnAO1tl~wOeHbY84EBGNfY5wRBvKYoeBAO9IbpkX5yzQg9ACi42Z40l8RZD797na9hvgIWqma5MYxK26UykfnEeaooOOjhDKzLsTm9izRevAahNSefhGQHZfaaDZZnEe32~Hz-TGsS0Ko1xf9MmNsk5ZWFkNV0J9hM8MaZpA82hieEHPDCzquwpPcQGLLEuPYVcVRw5ooARWT-qtsJgFDzp-U27pPSN4hNflFv30-at1aMtfeJayoe9ahangrXojChanl2Fz2Z8QGVCP60l84Af-UOjTxOPHWp2Icw2h5HFbR89A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. In vivo expression of VWF fragments elevates basal FVIII levels in Vwf−/− mice. Circulating VWF levels (A) and FVIII levels (B) were measured from 6 to 8 hydrodynamically injected Vwf−/− mice at the indicated time points (mean ± standard deviation [SD]). (A) VWF concentrations measured in the PPP were converted to molarity of subunits based on the molecular mass of each VWF fragment calculated from the amino acid sequence (wtVWF = 228 kDa, ∆Pro = 228 kDa, D1D3-GS-CK = 71 kDa, D1-D3 = 56 kDa, D′D3 = 56 kDa, truncD′D3 = 33 kDa). The black line corresponds to the VWF level in pooled PPP from 10 C57BL/6J mice (3.4 μg/mL). Preinjection VWF levels were undetectable by ELISA. Plasma levels were not measured for SS. The width of the error bars likely stemmed from the highly variable response to the hydrodynamic injection and subsequent recovery from this injury. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. d, day; wk, week.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/3/10.1182_blood-2013-11-540534/4/m_445f2.jpeg?Expires=1769686969&Signature=mDdjUL0pqqHQutl41~uRD4dlMS5HJiFpP7uxLElXq9RCZpPI2Vt~Zvyb82TS4U1RZQkDDM3mFmnBQunx~~O2hSozNMeQCBmcVVfTkAYJ7o7eViqtI~NOl8D1D0lfTA~7uyvFa8G8lxQpasaQfE8W8GOW5GCybQH9J3s7H3etsWVgQiVx504rvzV3qP9PKfOyK~8oy~0sdKHZn0mX7uV2HMCQzbNwMup2-Hp~UYL4GlKJZdbfuxU6yoBw~8WPAt8Ijc-h8uJj~46nJao-PKaBQN62q8zpwb97K0-Fx4GTcGr1EmDirNVYfjN5Kp8ldhJfY7bMSkqUGROBry0N28xpzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Transfused VWF fragments exhibit t1/2 of ∼1 to ∼4 hours (hrs). PPP isolated from hydrodynamically injected Vwf−/− mice (n = 5-11 mice per VWF fragment) by cardiac puncture was pooled and transfused into 4 to 9 naive Vwf−/− mice. Residual VWF levels (mean ± SD) in recipient mice were normalized to the 5-minute time point (set as 100% [%5min]). Measurements were nonlinearly regressed to predict the clearance of each FVIII-stabilizing VWF fragment (curves).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/3/10.1182_blood-2013-11-540534/4/m_445f3.jpeg?Expires=1769686969&Signature=eRJ6e2D0z2RO2xiVgS2IIA9pYP9uVOelMiWo9qbFwmUikf2-qqDzTD7xQvsIgnWY1I5PHi2iFblF97LXgBI3WrxY7uxrzz-7xAt70aSaEacCYy93em3Itozu4iWMhKUSkIJPUeeCEghoNPBXPppQZmttoF14lFLxPC5eVw7PmyeJzSGuM1GcTpUHzVRDwyow9w9RqL4FhI7KQnJgYhCn~ij-jwO8XaCuVk3xW9nRwZ7xB4O5cDz4ogxgaBUj5eOBBvnL9ga-MTcAU2HlBWm5ZizOV1XFZcc72wqW3c~qvtRGb8PKWU36SLjrmsixhgJbVBqan7A32ayVffUYYUjyuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. D′D3-Fc is retained in the circulation and elevates FVIII plasma levels for >7 days in Vwf−/− mice. VWF fragment levels (normalized to the value at 5 minutes as 100% [%5min], A) and FVIII levels (B) were followed in Vwf−/− mice (n = 4-5) transfused with 200 μL pooled PPP derived from hydrodynamically injected Vwf−/− mice (n = 5-7). Donor PPP composition (VWF in nM of subunit, FVIII in %C57BL/6J): D′D3-Fc (9.5 nM, 41.8%), D′D3 (4.9 nM, 67.2%), and wtVWF (49.7 nM, 108.6%). (A) VWF levels for wtVWF and D′D3 shown here are from a subset of mice included in Figure 3 whose FVIII levels were also measured. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. The mean pretransfusion FVIII levels from all treated Vwf−/− mice (n = 14) are indicated by the black line (8.5%). Data (mean ± SD) were nonlinearly regressed (curves).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/3/10.1182_blood-2013-11-540534/4/m_445f4.jpeg?Expires=1769686969&Signature=z7ecrUXlnARe8-aLFGVBbTHvo6rg2jves-oJTAn1Rc48QMTu~ofWcwzKM6ZYLXdivKHKgBruJXmjgKP0DgKokf8ITvtlubr9LnZMIbJo4ngXmW6fHbobhcnrI0oztTX0DvbdaiKbsdSEbJ~l5fXCoEvkqWCthA6fqjaWxVZhVuGDkMQn323fPcG7V9spNH92qNqQDTIODIBR87HPKqscP8G9-OVWPYA3VpPsutBJd89ogz4encdVY4MPlmv5SB5JtIwUkdrJyWGTEbZPvxnAatjnsLcaKFTQZi0A0Z3ufttC6y363-9VFIqCz2K5REj0WRfgTKyL7Cv42TyEpXVOxA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Fc fusion prolongs D′D3 but not FVIII survival in HA mice. VWF fragment levels (normalized to the value at 5 minutes as 100% [%5min], A) and FVIII levels (B) were followed in HA mice (n = 4-9) transfused with 200 μL pooled PPP derived from hydrodynamically injected Vwf−/− mice (n = 5-6). Donor PPP composition (FVIII in %C57BL/6J, VWF in nM of subunit): wtVWF (122.6%, 15.5 nM), D′D3 (71.0%, 3.4 nM), and D′D3-Fc (90.0%, 3.2 nM). Data (mean ± SD) were nonlinearly regressed (curves) as a single-phase exponential decay. (B) FVIII levels are expressed as %C57BL/6J, where 100% is defined as the amount of FVIII in PPP pooled from 10 C57BL/6J mice. The FVIII levels in mice transfused with D′D3-Fc were not regressed because of insufficient time points; a line is drawn through the data points for visual assistance.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/3/10.1182_blood-2013-11-540534/4/m_445f5.jpeg?Expires=1769686969&Signature=kM7Di~SGpj7HAfoC5Glh1S5K~HKEoRCVtZ1WNixMr-HqW0pV4AlalooBWbFPJVV2N1z8oHHoo5T6W6awq8bRYJuc2o0Mp8FCQ3IfEDR4nvHVlrB5qREvfFlerRlYNAyzwwASN3YP15pgAsFPJFht4bruJOu0CgFw17-GWBFgjnQcsHjyxTpqxlcVqWAjapF37KTGrkZLFGp~Hgx-PakbS8~ym7ueppC7kYW3fxTzZ91WipDkKbyl1eji6~7NmUt2wqOzXsPW9aitMuecX4PH0CFentbpQjm1TA3ul0dWuiCf9wvclDXsxF4~3LlzIYmsv053cokWcS2Rq~WyYuEHzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)