In this issue of Blood, Stemberger et al show that the progeny of a single CD62Lhi cell can provide protection against bacterial challenge and that ultra-low doses of cytomegalovirus (CMV)-specific CD8+ T cells can expand greatly in humans.1
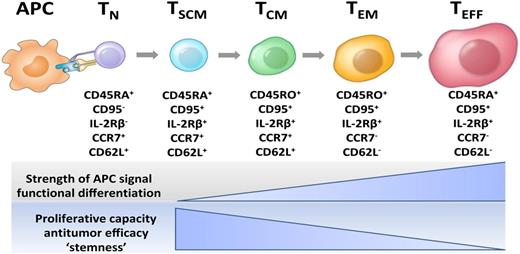
Current model of CD8+ T-cell central and effector memory generation for mouse and human T cells. This model of T-cell differentiation is also known as the “linear” or “developmental” model. Unlike previous models that proposed that T effector cells gave rise to memory cells, the developmental model indicates that less differentiated memory cell types that express CD62L give rise to effector cells, and not vice versa. APC, antigen-presenting cell; TCM, central memory T cell; TEFF, effector T cell; TEM, effector memory T cell; TN, naive T cell; TSCM, T memory stem cell. Figure prepared by Dr Nicholas P. Restifo.
Current model of CD8+ T-cell central and effector memory generation for mouse and human T cells. This model of T-cell differentiation is also known as the “linear” or “developmental” model. Unlike previous models that proposed that T effector cells gave rise to memory cells, the developmental model indicates that less differentiated memory cell types that express CD62L give rise to effector cells, and not vice versa. APC, antigen-presenting cell; TCM, central memory T cell; TEFF, effector T cell; TEM, effector memory T cell; TN, naive T cell; TSCM, T memory stem cell. Figure prepared by Dr Nicholas P. Restifo.
Drug-based therapy has long been a mainstay of disease treatment, but cells are playing a broader role in modern medicine. The pharmacokinetics of drugs are often measured in months or days, but living cells can persist in the host for months and years and in many cases a lifetime. Classic experiments by Weisman showed that small numbers of hematopoietic stem cells were capable of saving 50% of lethally irradiated mice and of proliferating and differentiating into all blood cell types.2 What has become most surprising is that stem cell–like properties exist in a wide variety of adult human cells including in the gut, skin, and even in the central nervous system. In addition, qualities of “stemness” (multipotency and self-renewal) exist in cellular compartments not previously expected to contain stem cell–like cells, such as in certain subpopulations of postthymic T cells.3,4
In this issue of Blood, Stemberger et al observe that cells contained within the CD62Lhi populations of CD8+ T cells have the stem cell–like property of persistence, confirming earlier observations.5 The finding that CD62Lhi antigen-specific T cells are more persistent than more differentiated CD62Llo cells is consistent with linear models of T cell differentiation.6,7 Thus less differentiated or “younger” memory cells developmentally precede effector cells (see figure). The observation that CD62Llo cells do not engraft strikes a blow to the notion that more differentiated effector cells give rise to memory cells as has been purported by Ahmed and colleagues.8
The clear advantage of using small numbers of T cells with stem cell–like properties is that they are likely to be oligoclonal, minimizing graft-versus-host disease.1 In an interesting extension of this mouse work, Stemberger et al treated patients that had received an allogeneic hematopoietic stem cell transplant (HSCT) with low doses of streptamer-enriched human CMV-specific CD8+ T cells and found robust pathogen-specific T-cell expansion. The authors obtained some interesting clinical correlates to these data suggesting that tiny numbers of administered cells (∼5000 cells per kg) were clinically effective.
If one thinks of these cells as a drug, the drug would be administered as a single, one-time dose that is very small indeed. If 109 T cells weigh 1 g, then 5000 T cells per kg would be the equivalent of giving a single dose of a drug at just 5 μg/kg. Cells are “living therapy” capable of expanding and persisting for the life of the host. The authors do not break down the subtype of the cells that they infuse, but it is interesting to speculate that the cell infusions were composed of some component of postthymic stem-like T cells. The doses of highly effective cells might be very small indeed, and it seems plausible that even a single T cell might be able to repopulate an individual and trigger lifelong protection from specific viruses and even cure large, established cancers.
Antiviral T cells can come in numerous cell subtypes, including CD62L+ T memory stem cells (TSCM), a subset capable of massive proliferation and indefinite persistence. Although it is too early to say whether stem cell–like populations of lymphocytes will be highly effective as approved treatments for cancer or infectious diseases, the properties of human TSCM have been recently elucidated.9 The advantage of treating lymphodepleted hosts, including patients postallogenic HSCT, is that high levels of circulating “homeostatic cytokines” such as interleukin-7 and interleukin-15 can trigger robust T-cell proliferation.10
These efforts should now be expanded in order for promising but still unapproved cellular therapies to be become part of medical practice. Trials must be done to treat patients beyond the still-small patient cohorts that have entered into clinical trials and beyond the very few diseases that have experimentally treated to date. Academic and commercial investment must be devoted to culturing good manufacturing practice–quality cells for infusion into patients to increase capacity.
A great deal of basic science will be required to improve the use of cellular therapies. A growing array of biological tools is increasingly available to the biomedical research community that allows manipulation and transfer of cells or tissues, autologous or allogeneic, genetically engineered and/or epigenetically reprogrammed to treat or prevent disease. Gene modification might enable the improvement of cell-based therapies, and these include not just the addition of new genes but their removal as well.
The possibilities for application of emerging biological concepts in epigenetics, regenerative medicine, cellular reprogramming, immunotherapy, and genetic engineering for treatment of dread disease are innumerable. Unlike drugs, which require major investment from pharmaceutical companies, progress in cell-based therapies has thus far been driven largely by academics. New cell therapies could soon be in use for the treatment of infectious diseases, cancer, immunodeficiencies, monogenic disorders, and degenerative diseases. With Stemberger et al’s novel confirmation of the big bang theory of stem cell–like T cells, the universe is likely be a rapidly expanding place.
Conflict-of-interest disclosure: The author declares no competing financial interests.