To the editor:
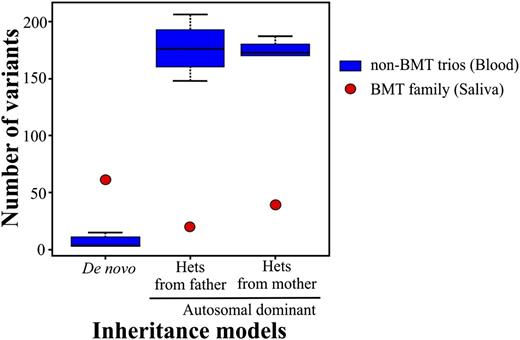
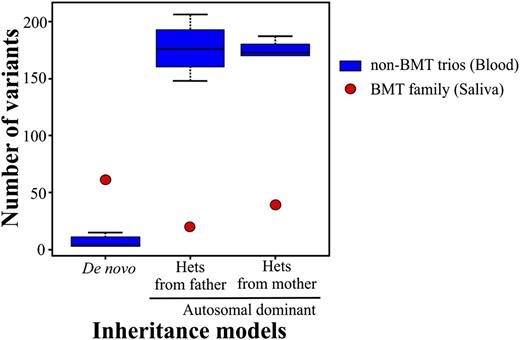
We routinely perform clinical exome sequencing on blood samples from patients without bone marrow transplants (BMTs). Moreover, we accept saliva samples due to the acceptable DNA quality, noninvasiveness, and improved percentage of participants.1 We assess the quality at the wet laboratory as well as the bioinformatics process which includes monitoring the number of variants after inheritance model filtering. Recently, a 4-member family (mother, father, female proband, and affected sister) was referred for exome analysis due to combined immune deficiency of undetermined genetic etiology in the proband. The samples that were submitted for analysis were blood for both parents and saliva for both affected sisters. All of the quality controls passed the set thresholds except for the inheritance model variants which deviated from their normal range. Specifically, the number of de novo variants was inflated, whereas the opposite was true for the autosomal-dominant variants (Figure 1). We hypothesized that a switch of DNA samples had occurred either during collection or processing in the laboratory. Short tandem repeat (STR) analysis confirmed that the relationship among family members was correct, however, unidentified peaks at all STR loci were present in both sisters, including the presence of a Y chromosome peak suggesting contaminating male DNA. This confirmed that the variant number discrepancy of the inheritance models can be attributed to the male DNA contaminating the proband’s sample. Upon further investigation with the referring physician, it was indicated that both sisters had undergone BMTs from 2 distinct unrelated male donors.
Box-and-whisker plot of the number of variants per exome trio for de novo and autosomal-dominant inheritance models. The number of variants for the BMT trio significantly deviates from the normal range of non-BMT trios. The non-BMT control trios were randomly chosen from a group of 25 trios and include 15 individuals in 5 unrelated non-BMT trios. The bars represent the greatest and least values excluding the outliers. The values at the top and bottom of the boxes represent the upper and lower quartiles, whereas the line indicates the median.
Box-and-whisker plot of the number of variants per exome trio for de novo and autosomal-dominant inheritance models. The number of variants for the BMT trio significantly deviates from the normal range of non-BMT trios. The non-BMT control trios were randomly chosen from a group of 25 trios and include 15 individuals in 5 unrelated non-BMT trios. The bars represent the greatest and least values excluding the outliers. The values at the top and bottom of the boxes represent the upper and lower quartiles, whereas the line indicates the median.
Several XY fluorescence in situ hybridization (FISH) studies showed that a small percentage of bone marrow donor-derived cells can repopulate every part of the gastrointestinal tract (0%-4.6%), liver (0%-7%), and epithelial cells (0.2%-7.3%) in humans.2-4 However, other XY FISH studies reported higher levels of donor cells in buccal (0.8%-12.7%), liver (4%-43%), mouthwash (25%), and fingernail (8.9%-72.9%) samples.5-8 An STR study reported 25% of donor leukocytes in mouthwash cell pellets from BMT patients.5 Similarly, DNA extracted from fingernail samples of BMT patients showed coexistence of the donor pattern of the STRs (8.9%-72.9%).8 Consistent with 3 studies that showed high levels (>25%) of donor, based on STR analysis, our saliva samples had 61% and 28% and cytobrushes had 37% and 12% of donor in the proband and sister, respectively. Additionally, blood XY FISH analysis of the proband and sister revealed an incidence of 99% and 62%, respectively.
Several points should be noted. Saliva samples may have a higher level of donor cells than anticipated that will interfere with genotyping assays including exome sequencing. Thus, the level of donor chimerism must be established by STR analysis prior to genetic testing. Second, the inheritance modeling used in the exome analysis pipelines is sensitive to sample switching and contaminating DNA. Therefore, the importance of quality-assurance measures at each step of a complex exome test is critical to ensure accuracy. Third, fibroblast cells should be the sample choice for genetic testing of BMT patients, otherwise exome sequencing results may be misleading if saliva samples or buccal samples are processed.
Authorship
Contribution: C.A.V. and K.Z. designed research, analyzed and interpreted data, and wrote the manuscript; S.R.I., S.D., and J.C. collected data; A.M. and C.W. analyzed and interpreted data; J.C.B. collected, analyzed, and interpreted data; and I.C. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: C. Alexander Valencia, Division of Human Genetics, Cincinnati Children’s Hospital Medical Center, College of Medicine, University of Cincinnati, 3333 Burnett Ave, Cincinnati, OH 45239; e-mail: alexander.valencia@cchmc.org.