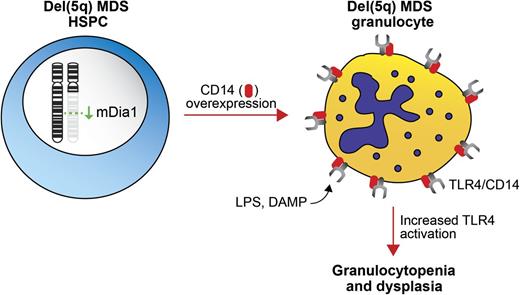
Model of DIAPH1/mDia1 deletion in del(5q) MDS. Deletion of mDia1, a chromosome 5q gene, results in overexpression of CD14 in granulocytes. Overexpression of CD14, a coreceptor for TLR4, leads to increased TLR4 signaling in granulocytes, granulocytopenia, and BM dysplasia.
Model of DIAPH1/mDia1 deletion in del(5q) MDS. Deletion of mDia1, a chromosome 5q gene, results in overexpression of CD14 in granulocytes. Overexpression of CD14, a coreceptor for TLR4, leads to increased TLR4 signaling in granulocytes, granulocytopenia, and BM dysplasia.
Deletion of chromosome 5q (del[5q]) is the most common cytogenetic alteration in MDS. Although minimally deleted regions and common breakpoints have been mapped in del(5q) MDS, all the relevant haploinsufficient genes have not been characterized.2 Adding to the complexity of the del(5q) MDS phenotype, it is becoming apparent that multiple genes contribute to the clinical manifestation of del(5q) MDS. As such, a systematic analysis of relevant chromosome 5q genes and their role in hematopoietic stem/progenitor cell (HSPC) function is warranted.
DIAPH1, which encodes mDia1, is located on 5q31.3, which resides between the 2 commonly deleted regions in del(5q) MDS. mDia1 is a RhoA GTPase effector that belongs to the formin protein family and plays an important role in linear actin polymerization.3 To mimic loss of mDia1 in del(5q) MDS and to understand the potential consequences of mDia1 deficiency on hematopoiesis, mDia1 knockout mice were created and analyzed. The mDia1-deficient mice exhibit a striking age-dependent granulocytopenia, which is cell autonomous as reduced granulocytes also occur in wild-type mice transplanted with mDia1-deficient bone marrow (BM) cells (see figure). Consistent with a role in MDS, mDia1-deficient mice also have prominent myeloid dysplasia in the BM.
Analysis of myeloid markers on the surface of mDia1-deficient BM revealed high expression of CD14, a coreceptor for the Toll-like receptor 4 (TLR4) and MD-2 complex, which detects bacterial lipopolysaccharide (LPS) (see figure). Interestingly, CD14 overexpression in mDia1-deficient mice is limited to granulocytes and committed granulocytic progenitors. The reason for the selective overexpression of CD14 in granulocytes is not known, yet it may be attributed to the restricted function of mDia1 to certain hematopoietic cell subsets, such as granulocytes. Importantly, granulocytes from del(5q) MDS patients also overexpress CD14, which inversely correlates with mDia1 expression, implying a direct role of mDia1 regulation of CD14.
Although mDia1-deficient mice develop granulocytopenia in a cell-autonomous manner, it was investigated whether exposing mice to low levels of LPS would exacerbate the phenotype. Indeed, mDia1-deficient mice developed an accelerated granulocytopenia with LPS treatment, underscoring the connection between loss of mDia1 and increased innate immune signaling. In addition to LPS, the TLR4/MD2/CD14 complex also recognizes host damage-associated molecular pattern molecules (DAMPs), which are produced by dying cells.4 Because increased apoptosis is a hallmark of low-risk MDS, it is conceivable that DAMPs released from dying cells are activating innate immune signaling within del(5q) MDS HSPCs and in turn, further contributing to the MDS phenotype.
Increased innate immune signaling has been previously reported in MDS. The first genetic evidence that increased innate immune signaling contributes to the pathogenesis of del(5q) MDS originated from observations involving loss of miR-146a,5 a microRNA within the deleted region on chromosome 5q. Knockdown or knockout of miR-146a in mouse HSPCs results in hematologic abnormalities resembling MDS, including impaired HSC function, peripheral blood cytopenia, and BM dysplasia, in part due to derepression of its target, tumor necrosis factor receptor–associated factor 6 (TRAF6).5,6 TRAF6 is a key signaling mediator downstream of TLR4. Given that mDia1 and miR-146a reside within 20 Mb of each other on chromosome 5q and are concomitantly deleted in del(5q) MDS, it will be imperative to create mice in which both genes are deleted. The primary hematopoietic defects associated with mDia1 deletion seem to be limited to granulocytes, whereas the defects following miR-146a deletion involve multiple hematopoietic cells, including long term HSC.7 It is possible that the effects on long term HSC will not be exacerbated; however, the granulocytopenia might be significantly worsened in the double mDia1/miR-146a knockout mice. The molecular mechanisms resulting from chronic innate immune signaling are not entirely clear. Although TLR4/TRAF6 signaling activates nuclear factor–κB (NF-κB) under acute stimulatory conditions, it is still not known whether other pathways play a role following chronic activation. Notably, gene expression profiling of granulocytes from mDia1-deficient mice did not reveal increased expression of NF-κB target genes, except following LPS stimulation, suggesting that chronic innate immune activation may affect additional molecular and cellular components that contribute to del(5q) MDS.
The importance of the innate immune pathway in MDS was recently revealed by use of small-molecule inhibitors that target the TLR4 pathway.8 Interestingly, lenalidomide, the mainstay therapy for del(5q) MDS, was shown to extend the lifespan of mDia1-deficient mice and reduce the expression of CD14. Although the mechanism resulting in reduced CD14 expression is not known, some of the beneficial effects of lenalidomide in MDS may occur through “tuning down” innate immune signaling.
Innate immune signaling in HPSCs and mature immune cells is a tightly controlled process that involves many checks and balances. In contrast, inefficient regulation of the innate immune signaling pathway in HSPCs, such as by deletion of negative regulators (miR-146a) or overexpression of positive regulators (CD14, TRAF6, TLRs), contributes to defects associated with MDS. Deregulation of innate immune signaling could be a driving force in the pathogenesis of MDS. Additional investigation into the signaling pathways and creation of new mouse models will deepen our understanding and potentially uncover novel therapeutic options.
Conflict-of-interest disclosure: The author declares no competing financial interests.