In this issue of Blood, Helton et al highlight the neuroradiological findings of children enrolled in the Strokes With Transfusions Changing to Hydroxyurea (SWiTCH) trial.1 This study, which screened 161 children with sickle cell anemia (SCA) receiving chronic transfusion therapy for prevention of recurrent strokes, is the largest SCA cohort followed prospectively with magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) published to date, giving additional insights into the relationships between strokes and cerebral vasculopathy in SCA.
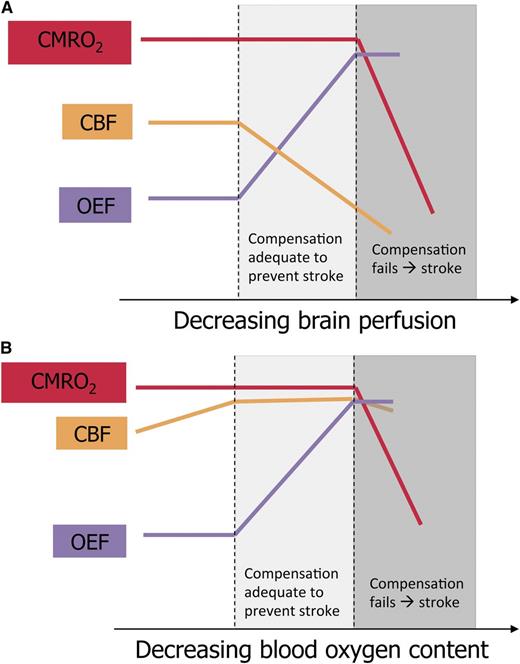
Schematic representation of brain perfusion, oxygen extraction, and oxygen metabolism in children with SCA. (A) Proposed model of cerebral infarction in SCA with cerebral vasculopathy. CBF declines as vasculopathy progresses. The percentage of oxygen extracted from the blood by the brain (OEF) increases to maintain adequate tissue oxygen utilization (CMRO2). When CBF continues to fall and OEF cannot increase further, CMRO2 drops, causing cerebral infarction. (B) Proposed model of cerebral infarction in SCA without cerebral vasculopathy. CBF is elevated to compensate for decreased blood oxygen content and has limited additional capacity to increase. As blood oxygen content falls due to acute illness, OEF increases to maintain adequate CMRO2. When OEF is maximized, a drop in CBF may cause CMRO2 to fall, and infarction ensues. The figure has been adapted from Powers4 with permission.
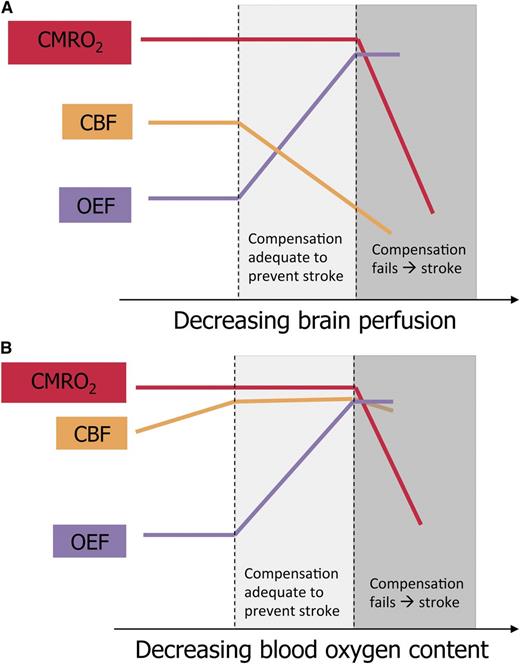
Schematic representation of brain perfusion, oxygen extraction, and oxygen metabolism in children with SCA. (A) Proposed model of cerebral infarction in SCA with cerebral vasculopathy. CBF declines as vasculopathy progresses. The percentage of oxygen extracted from the blood by the brain (OEF) increases to maintain adequate tissue oxygen utilization (CMRO2). When CBF continues to fall and OEF cannot increase further, CMRO2 drops, causing cerebral infarction. (B) Proposed model of cerebral infarction in SCA without cerebral vasculopathy. CBF is elevated to compensate for decreased blood oxygen content and has limited additional capacity to increase. As blood oxygen content falls due to acute illness, OEF increases to maintain adequate CMRO2. When OEF is maximized, a drop in CBF may cause CMRO2 to fall, and infarction ensues. The figure has been adapted from Powers4 with permission.
Cerebral vasculopathy in children with SCA is a significant predictor of first2 and subsequent3 strokes and progresses despite chronic transfusion therapy.3 The SWiTCH participants had a significant burden of vasculopathy: 69% of children demonstrated arterial stenosis at study entry.1 Children with more severe vasculopathy were more likely to have low or uninterpretable transcranial Doppler ultrasound (TCD) velocities in the cerebral arteries, reinforcing that cerebral blood flow through these arterial territories is likely severely compromised. Not surprisingly, children with more severe arterial stenosis had a greater likelihood of cortical and subcortical infarctions. Furthermore, during the study period, 16% of the participants had evidence of brain ischemia: 7 children experienced recurrent overt strokes, 1 had a silent infarct, and 17 had transient ischemic attacks.
SWiTCH’s primary aim was to compare the efficacy of substituting hydroxyurea therapy plus serial phlebotomy vs standard therapy of transfusions plus chelation on a composite end point of preventing strokes plus reducing iron overload. The study was terminated early due to predicted futility of the intervention on the composite end point, but the neuroimaging data shed light on the severity of cerebrovascular disease in children with SCA and strokes. Most importantly, how can clinicians use this information to target more effective therapies to high-risk children?
Children with SCA receiving transfusions for stroke prevention are typically followed with serial MRI/MRA and may not undergo conventional angiography due to the potential risks of anesthesia and intravenous contrast agents. Helton et al developed an MRA-based vasculopathy grading scale1 that they plan to validate in the ongoing TCD With Transfusions Changing to Hydroxyurea (TWiTCH) study. A standardized, validated SCA-specific grading scale would give hematologists a common language with which to describe vasculopathy in our patients. Although TCD is validated for detecting children at high risk for a first stroke, no tool currently exists for predicting strokes while receiving transfusion therapy, so validation of this scale is clearly needed.
For a deeper understanding of stroke pathophysiology in SCA, however, we should look beyond TCD blood flow velocities and vascular stenosis seen on angiography to tissue-level cerebral blood flow (CBF) and regional oxygen metabolism. The cerebral metabolic rate of oxygen consumption (CMRO2) is determined by the product of CBF, the fraction of oxygen extracted from blood (OEF), and blood oxygen content (CaO2). There is scant published literature about cerebral oxygen metabolism in SCA, but parallels may be drawn between children with SCA vasculopathy and adults with carotid atherosclerosis and ischemic stroke, about whom much more is known. Positron emission tomography (PET) studies in these adults demonstrate that the initial compensatory response to perfusion pressure limitation is vasodilation of cerebral arterioles to preserve CBF, termed autoregulation. If perfusion pressure decreases beyond maximal compensatory vasodilation, OEF increases as a second compensatory mechanism to maintain constant CMRO2. If perfusion pressure continues to fall, both compensatory mechanisms can no longer maintain the CMRO2 required for tissue survival, and cerebral infarction ensues4 (panel A).
Although the pathophysiological mechanisms described above likely apply to children with cerebral vasculopathy, SCA imposes additional physiological challenges. First, CaO2 is reduced due to anemia, inducing compensatory increases in baseline CBF.5 Second, individuals with SCA have limited autoregulatory cerebral vasodilation because of their baseline CBF elevation.5 Third, the increased viscosity of hemoglobin S-containing blood under low-shear conditions further limits tissue oxygen delivery and persists despite transfusion therapy for stroke prevention.6 Additional physiologic stressors, such as decreased CaO2 due to acute worsening of anemia or hypoxemia, decreased CBF due to acute hypotension, or increased tissue oxygen demand due to febrile illness, may lead to inadequate CMRO2 and thus a stroke (panel B). Thus, children with SCA and vasculopathy are exposed to the double jeopardy of flow-limiting stenosis and underlying SCA physiology.
Only 2 studies in children and adults with SCA have used PET plus MRI to characterize cerebral infarcts. Both found areas of decreased blood flow and glucose metabolism on PET that correlated with, and in some cases were more extensive than, MRI-identified infarcts.7,8 While demonstration of existing brain injury is important, a means of predicting future stroke risk would be even more beneficial for patients. PET studies have become undesirable in children because of the risks of ionizing radiation exposure. Thus, safer imaging techniques, such as novel MR-based imaging, are needed to further characterize cerebral oxygen metabolism in children with SCA and to identify those children in greatest need of brain-preserving intervention.
A comprehensive model of the mechanisms of strokes in children with SCA ultimately will guide the development of better therapeutic strategies. Clearly, mitigating anemia with blood transfusion therapy3 or hydroxyurea1 does not prevent all recurrent overt or silent infarctions or transient ischemic attacks, especially with coexistent cerebral vasculopathy. Allogeneic hematopoietic stem cell transplantation has produced better results, preventing infarctions in children with successful engraftment after the immediate post-transplant period.9 However, most patients with SCA lack an HLA-identical sibling donor, and alternative donor transplantation strategies remain experimental. Surgical revascularization, widely applied in idiopathic moyamoya disease and cerebral vasculopathy due to other conditions such as neurofibromatosis or trisomy 21, has been reported in several SCA case series with resulting clinical improvement,10 but changes in CBF and OEF following revascularization have not been investigated. To determine the benefits of transfusion therapy, hydroxyurea, hematopoietic stem cell transplant, or surgical revascularization, we need to measure changes in brain perfusion and oxygen utilization. With a better understanding of stroke pathophysiology in children with SCA, we can develop more effective treatments to prevent sickle cell’s devastating neurological consequences.
Conflict-of-interest disclosure: The authors declare no competing financial interests.