In this issue of Blood, Myneni et al demonstrate a role for the A (transglutaminase) subunit of factor XIII (FXIII-A) in cell culture models of differentiation of preadipocytes to adipocytes.1 This finding is potentially of great importance in light of recent genome-wide association and adipose tissue transcriptomic studies that implicated F13A1 in human obesity.2
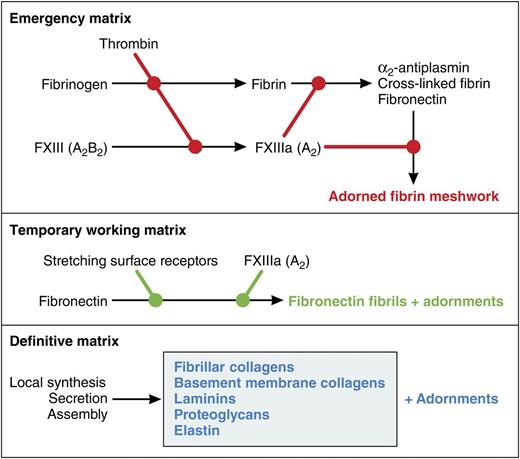
Conceptual model of roles of FXIII-A in the formation of extracellular matrices. FXIII-A is best known as being activated by thrombin and covalently cross-linking and stabilizing the emergency matrix of fibrin formed when the blood coagulation cascade is activated. Cells undergoing growth and differentiation or responding to injury assemble fibronectin into fibrils that constitute a temporary working matrix and are used for multiple purposes depending on the cell type. Assembly is catalyzed by surface receptors that stretch fibronectin. In preadipocytes, assembly is enhanced by the FXIII-A. Finally, differentiated cells have a unique definitive matrix suited to their function. Each of the matrices has the potential to be adorned with other proteins. Cross-linking catalyzed by FXIII-A may contribute to some of the adornments. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conceptual model of roles of FXIII-A in the formation of extracellular matrices. FXIII-A is best known as being activated by thrombin and covalently cross-linking and stabilizing the emergency matrix of fibrin formed when the blood coagulation cascade is activated. Cells undergoing growth and differentiation or responding to injury assemble fibronectin into fibrils that constitute a temporary working matrix and are used for multiple purposes depending on the cell type. Assembly is catalyzed by surface receptors that stretch fibronectin. In preadipocytes, assembly is enhanced by the FXIII-A. Finally, differentiated cells have a unique definitive matrix suited to their function. Each of the matrices has the potential to be adorned with other proteins. Cross-linking catalyzed by FXIII-A may contribute to some of the adornments. Professional illustration by Patrick Lane, ScEYEnce Studios.
The A subunit is the most enigmatic of the blood coagulation cascade participants. It has the characteristics of a cytoplasmic protein—no signal sequence, no asparagine-linked glycosylation, and no disulfides.3 The mechanism for its secretion from cells is unknown. Once in the circulation, it binds to the B subunit, a proper plasma protein that is secreted classically by hepatocytes.4 The subunits form an A2B2 tetramer that circulates in plasma with a half-life of >1 week.
On activation of blood coagulation, the A subunit is cleaved by thrombin, releasing an activation peptide and allowing A2 to dissociate from the B subunits and become catalytically active (FXIII-A). FXIII-A covalently cross-links and stabilizes fibrin (see figure). FXIII-A also cross-links circulating α2-antiplasmin and fibronectin into the fibrin meshwork; the former presumably protects the meshwork from incidental lysis, whereas the latter facilitates cellular ingrowth and subsequent healing. Deficiency of plasma FXIII from the lack of either the A or B subunit is associated with a distinctive spectrum of problems, including umbilical bleeding during the neonatal period, delayed soft tissue bruising, mucosal bleeding, life-threatening intracranial hemorrhage, poor wound healing, and recurrent miscarriages.5
Although the sources of circulating A2 have not been explored thoroughly, megakaryocyte/platelets and monocyte/macrophages likely are major players. Message for the A subunit, however, is found in a variety of cell types, raising the possibility that the A subunit may have cell type-specific functions independent of its role in blood coagulation. Myneni et al now describe a function for the A subunit synthesized during adipocyte differentiation.1 They demonstrate that the A subunit forms an active transglutaminase that translocates to the cell surface and promotes assembly of fibronectin into extracellular matrix, which in turn causes the differentiating cells to proliferate more in response to insulin, a key differentiation agent, while slowing down differentiation and accumulation of lipid. Two experimental paradigms implicate the A subunit in these events: inhibition by a small molecule called NC9 that incorporates irreversibly into transglutaminases and a comparative study of fibroblasts cultured from mice in which the A subunit had been knocked out.
Conditioning surfaces of culture tissues with adsorbed fibronectin has long been known to dampen adipocytic differentiation.6 The present paper focuses on a more physiologically relevant form of insolubilized fibronectin, ie, fibronectin that is assembled into extracellular fibrils. These fibrils are elaborated by many types of cells and in a variety of experimental setups allow cells to proliferate and differentiate in response to various cues. The figure depicts the fibronectin matrix as a temporary working matrix that is the precursor to the different types of definitive matrix laid down once cells are fully differentiated. One may also think of it as a replacement for the emergency matrix of fibrin formed when thrombin is activated. As with the fibrin matrix, the fibronectin matrix and various definitive matrices are adorned with other proteins.
Fibronectin assembles at specific sites on the surfaces of cells. These sites are regulated and engage fibronectin via its N-terminal type I repeats and more C-terminal integrin-binding sequences. The sites are linked to cytoplasmic motor proteins, allowing stretching of fibronectin and exposure of cryptic self-association sites.7 Fibronectin is cross-linked efficiently to binding partners by FXIII-A, which attacks glutamines near the fibronectin N terminus.8 However, cross-linking is not required for assembly. My laboratory found that increases in the rate of fibronectin deposition in response to exogenously added FXIII-A varied depending on the cell being studied: twofold for cultured human dermal fibroblasts and >12-fold for HT-1080 fibrosarcoma cells.9 In the present paper, endogenous A subunit was deduced to cause an approximate twofold increase in fibronectin deposition by fibroblasts differentiating toward adipocytes.1 It should be noted that a second transglutaminase, transglutaminase-2 (TG2), was present in preadipocytes. Although TG2 also attacks the N-terminal glutamines of fibronectin,8 it seemed to play no part in modulation of fibronectin assembly and adipocyte differentiation.
The hematological community has always welcomed the chance to think more deeply about FXIII.10 Many questions are raised by the present paper and the genetic studies in which 7 different single nucleotide polymorphisms implicate F13A1 in obesity.2 What are the limits of the F13A1 transcriptional unit? Comparing preadipocytes to other cell types such as megakaryocytes and monocytes, how is transcription regulated, what controls translation, and how is the A subunit processed and secreted? How does the A subunit associate with the preadipocyte cell surface? How is it activated? What proteins are cross-linked? Can any or all of the polymorphisms that implicate F13A1 as an obesity gene be tied to modulation of fibronectin assembly by the preadipocyte? Answers to these questions could help fight the current obesity epidemic while at the same time shedding light on enigmas about the A subunit that persist 70 years after the discovery of its action on clot solubility.
Conflict-of-interest disclosure: The author declares no competing financial interest.