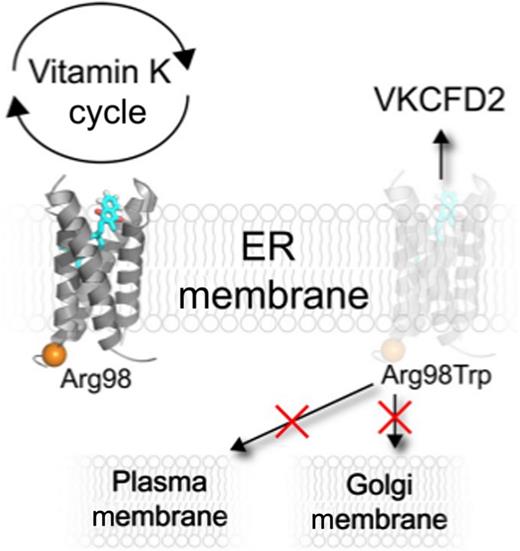
In this issue of Blood, Czogalla et al identify that the rare heritable hemorrhaging disease, vitamin K–dependent clotting factor deficiency type 2 (VKCFD2), is caused by mislocalization of vitamin K epoxide reductase (VKORC1) in the endoplasmic reticulum (ER).1
The enzyme VKORC1 reduces vitamin K that functions as a cofactor activating vitamin K–dependent proteins that are critical in coagulation. Czogalla et al show that Arg98 is crucial for proper ER localization of VKORC1, and that the VKCFD2 mutant Arg98Trp causes mistrafficking of VKORC1 from the ER with a concomitant reduction of ∼80% of VKORC1 levels in the ER membrane. Interestingly, tracking the Arg98Trp VKORC1 mutant shows that the protein does not accumulate in the Golgi or plasma membranes. These results highlight, for the first time, a potential mechanism as to why VKCFD2 patients have impaired coagulation capabilities.
The enzyme VKORC1 reduces vitamin K that functions as a cofactor activating vitamin K–dependent proteins that are critical in coagulation. Czogalla et al show that Arg98 is crucial for proper ER localization of VKORC1, and that the VKCFD2 mutant Arg98Trp causes mistrafficking of VKORC1 from the ER with a concomitant reduction of ∼80% of VKORC1 levels in the ER membrane. Interestingly, tracking the Arg98Trp VKORC1 mutant shows that the protein does not accumulate in the Golgi or plasma membranes. These results highlight, for the first time, a potential mechanism as to why VKCFD2 patients have impaired coagulation capabilities.
The enzyme VKORC1 is at the heart of the vitamin K cycle and coagulation, where it activates vitamin K for use by γ-glutamyl carboxylase in posttranslational modification of vitamin K–dependent clotting factors. Physiologically, VKORC1 is localized to the ER where it is the target of warfarin therapy, and mutations that affect this therapy have led to extensive personalized medicine studies.2 The major phenotype of VKCFD2 is spontaneous bleeding and diminished coagulation efficiency that can usually be controlled by oral supplementation of vitamin K. An unusual phenotype of VKCFD2 is that elevated quantities of the deactivated, oxidized epoxide form of vitamin K are found in the serum when patients are treated with vitamin K.3 This is in contrast to healthy individuals who have undetectable serum levels of deactivated vitamin K, even with significant vitamin K supplementation.
Currently, VKCFD2 has been exclusively associated with a single arginine to tryptophan mutation at residue 98 (Arg98Trp) in VKORC1.4 The vast majority of VKORC1 mutations that affect function, coagulation, or warfarin therapy are predicted to be clustered around the enzyme active site.5 This is in stark contrast to Arg98Trp which is predicted to be opposite the active site near the juxtamembrane region (see figure). Czogalla et al astutely recognized that arginine 98 is in proximity to another arginine at position 100, potentially forming an RXR-like diarginine motif. Membrane protein trafficking is complex and not fully understood; nonetheless, it has long been recognized that polybasic residues such as diarginine motifs, in membrane proteins like VKORC1, can function as an ER-localization signal.6-8 Utilizing confocal microscopy, Czogalla et al probed the effects of the VKORC1 VKCFD2 mutation on ER localization. They found that the p.Arg98Trp disease mutation causes a significant decrease in VKORC1 ER localization. A lack of VKORC1 ER retention is suggestive for the first time of a VKCFD2 pathophysiological mechanism that can explain impaired coagulation.
In addition to probing the effects of Arg98 on VKORC1 ER retention, the highlighted study also attempted to characterize the mistrafficking of the mutant VKORC1. The results show that the mutant does not aggregate in either the Golgi or plasma membranes, but curiously is consistent with membrane protein accumulation in the cytoplasm, presumably as an intermediate prior to degradation. A thorough analysis of polybasic VKORC1 regions also isolated an additional ER localizing domain composed of a dilysine domain in the C terminus that contributes to ER localization.
ER signals have been shown to be dependent on the identity, context, availability, and membrane proximity.6,7 An additional VKORC1 RXR motif, Arg33-Ala34-Arg35, was shown to play no role in ER retention. This is an interesting result given that there is controversy over the membrane topology of VKORC1. The contradicting models support either a 3-transmembrane or 4-transmembrane structure that is predominantly based on cell biology and biochemistry experiments or an x-ray crystallography structure of a distantly related bacterial protein, respectively.5 The 3- and 4-transmembrane helix models disagree on the orientation of the first transmembrane helix and whether a putative C-terminal region is a second transmembrane helix or forms part of a large ER luminal loop. These alternative models have important mechanistic and potentially therapeutic implications. The normal ER retention associated with Arg33-Ala34-Arg35 mutations is compatible with the 4-transmembrane VKORC1 model because it suggests that this region faces the ER lumen, whereas polybasic ER signaling motifs typically face the cytoplasm. Notwithstanding, one must not read too much into this result given that membrane proximity alone can influence the efficiency of a dibasic motif to localize to the ER membrane.7
Among a number of important unresolved questions regarding the vitamin K cycle is how vitamin K is regulated and shuttled to and from protein constituents such as VKORC1. As hinted at above, patients afflicted with and treated for VKCFD2 have unusually high levels of vitamin K epoxide in their serum. Although not addressed in this timely study, one wonders whether the appearance of vitamin K epoxide in serum from the vitamin K cycle is dependent on the absence of the VKORC1 from the ER membrane and whether an initial clue on vitamin K shuttling has been uncovered, or whether the observations are more convoluted. Regardless, future studies will build on these results that illustrate for the first time a potential mechanism for VKCFD2.
Conflict-of-interest disclosure: The author declares no competing financial interests.