Abstract
The development of curative systemic treatment of Hodgkin lymphoma was recently voted one of the top 5 achievements of oncology in the last 50 years (http://cancerprogress.net/top-5-advances-modern-oncology). The high expectation of cure (above 80%) with initial therapy, even for advanced disease, is tempered by the recognition of some important limitations: not all patients are cured, especially those in older age groups, and patients have suffered debilitating or, in some cases, fatal long-term side effects. The challenge for modern treatment approaches is to improve the cure rate and, at the same time, minimize the long-term damage resulting from treatment. After several decades during which we have tested a variety of different ways to combine conventional cytotoxic treatments with or without radiotherapy but have identified no effective new approaches, the field is once again moving forward. The developments that hold the greatest promise in this respect are the application of functional imaging with 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) to make an early judgment of the success of treatment and the introduction of some highly active new agents such as antibody-drug conjugates.
Introduction
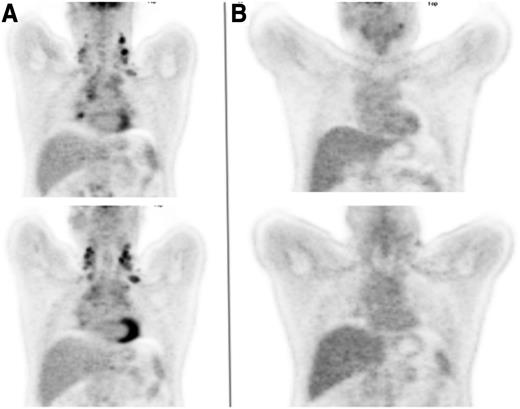
A 45-year-old man presented with progressively increasing breathlessness, a dry cough, drenching night sweats, and significant weight loss. Physical examination identified enlarged lymph nodes in both sides of the neck and both supraclavicular fossae. A chest radiograph showed widening of the mediastinum, which a computed tomographic (CT) scan confirmed as being the result of lymph nodes of maximum diameter 12 cm, accompanied by small pleural effusions, enlarged aortocaval nodes, and a focal defect in the spleen. Hemoglobin was 114 g/L, total white cell count was 9.2 × 109/L, lymphocyte count was 1.0 × 109/L, erythrocyte sedimentation rate was 48 mm/h, albumin was 31 g/L, and β2-microglobulin was 3.1 mg/L. A biopsy from one of the cervical nodes demonstrated grade 2 nodular sclerosing Hodgkin lymphoma. An FDG-PET scan (Figure 1A) demonstrated increased uptake in bilateral cervical, supraclavicular, mediastinal, subcarinal, paraesophageal, paraaortic and aortocaval nodes, as well as a focus of uptake in the left iliac bone, which had not been noted on the CT scan.
Coronal FDG-PET images of the thorax. (A) Prior to initiation of therapy and (B) following 2 cycles of ABVD.
Coronal FDG-PET images of the thorax. (A) Prior to initiation of therapy and (B) following 2 cycles of ABVD.
What baseline investigations are required?
After diagnosis by excisional or core needle biopsy, initial evaluation should include a thorough history and clinical examination, in addition to routine blood counts, erythrocyte sedimentation rate, lactate dehydrogenase, and a virology screen. A baseline chest x-ray should be performed. PET-CT imaging at baseline is advised, because it can lead to a change in stage in 10% to 30% of patients.1 This also enables greater accuracy when this modality is used in subsequent response assessment. Although no large-scale prospective studies have been carried out, the sensitivity (87.5% to 100%) and specificity (86.7% to 100%) of PET-CT for detecting bone marrow involvement2-4 means that bone marrow biopsy need no longer be part of routine staging. Cardiac function should be evaluated with an echocardiogram in those older than age 65 years or those with risk factors for cardiovascular disease such as diabetes mellitus or hypertension. Pulmonary function tests should be performed in those with a history of respiratory disease or with risk factors such as a history of heavy smoking. We would not routinely repeat cardiac or pulmonary function testing during the course of treatment.
All postpubertal men should be offered sperm storage at diagnosis. In women, oocyte or embryo cryopreservation should be discussed prior to regimens containing significant doses of alkylating agents. After treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), one third of males have transient azoospermia, but almost no females experience early ovarian failure; the risk is much greater with alkylating chemotherapy.5
What is the best initial therapy for advanced disease?
The original observation that advanced Hodgkin lymphoma could be eradicated with 4-drug mechlorethamine, vincristine, procarbazine, and prednisolone (MOPP) combination chemotherapy6 was followed by 3 decades of experimentation with different cytotoxic regimens. The ABVD combination devised by Bonnadonna in the 1970s was shown to be a significant improvement with better disease control and less long-term toxicity,7 but further refinements using multiagent alternating, hybrid,8-10 dose-intense,11,12 and even myeloablative regimens13,14 failed to improve the results further, despite their theoretical appeal based on various mathematical models of tumor responsiveness. The one apparent exception to this was the escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone (eBEACOPP) regimen devised by the German Hodgkin study group (GHSG), which showed superior disease control and overall survival (OS) compared with an alternating regimen of cyclophosphamide, vincristine, procarbazine, and prednisone (COPP) and ABVD.15,16 Subsequent randomized studies have shown that eBEACOPP results in better initial tumor control than ABVD,17-19 but it has been harder to show an OS benefit, mainly because second-line therapy for the minority of patients whose disease recurs after ABVD therapy is very often successful, whereas salvage after failure of eBEACOPP is less reliable, and in at least one study, the treatment-related mortality was higher.18 A recent meta-analysis suggested some survival benefit (about 7% at 5 years) from initial treatment with eBEACOPP.20
The main deterrent for the use of eBEACOPP for all patients is its toxicity. Many will be rendered infertile and suffer from prolonged severe fatigue.21 All patients experience severe myelosuppression, and a small number develop secondary myelodysplasia or acute leukemia.22 The regimen is too toxic for use in those older than age 60 years.23 Although there is no excess of treatment-related mortality, two-thirds of patients experience grade 3 to 4 infections, including febrile neutropenia,24 resulting in high rates of hospitalization during treatment at significant cost. Administration of growth factor is necessary with each cycle at an additional financial burden. By contrast, ABVD is much less toxic: the majority of patients will retain their fertility,25 and there does not appear to be any excess of leukemia or other second malignancies.
An important aspect of using ABVD is that the dose intensity should be maintained. Many patients will experience myelosuppression, particularly neutropenia, but it has been shown that if the patients have good performance status and no significant comorbidity, it is safe to continue with treatment at full dose and on schedule, irrespective of the neutrophil count.26 We do not routinely use growth factors, given the febrile neutropenia rate of less than 10%,27 but they can safely be given when indicated. Suggestions that they may exacerbate lung toxicity were not supported in a small pilot study of polyethylene glycol-filgrastim with ABVD, in which no excess lung toxicity was observed.28
There is no good predictive test for bleomycin-related lung toxicity in those with normal baseline pulmonary function tests. Vigilance is required during treatment, and those who develop respiratory symptoms or who have a severe cutaneous reaction should be carefully evaluated, if necessary, with high-resolution CT scans of the thorax. Bleomycin can be dose-reduced or omitted altogether if there is evidence of abnormal infiltrates.
Do baseline prognostic features help to guide therapy?
An analysis of the correlates of progression-free survival (PFS) among more than 5000 patients with advanced Hodgkin lymphoma treated mainly during the 1980s yielded a 7-factor score that was able to discriminate groups of differing outcome, although less than 10% were identified in the worst category.29 If anything, the results of similar analyses in the more recent past have shown a progressive improvement in the outcomes, so that the worst 7% of patients now show a 62% freedom from progression at 5 years.30 This makes it hard to define a group with sufficiently poor prognosis a priori to require a different approach to treatment, and studies that have tested the intensification of therapy for those in the higher-risk groups have all shown negative results. The patient in the case described had a prognostic score of 3, giving a predicted freedom from progression of 60% in the original model or 74% in the more recent analysis from the British Columbia Cancer Agency.
The difficulty of applying purely clinical prognostic factors has led to the exploration of biomarkers which may better reflect the underlying biology of Hodgkin lymphoma. These include circulating cytokines,31-33 chemokines,34 and soluble receptors such as CD30,35 as well as analyses of the tumor itself by gene expression profiling36,37 or enumeration of infiltrating macrophages.38 All of these are potentially interesting areas for research but are as yet unproven and in need of prospective validation, especially those that depend on immunostaining, which are generally subject to poor reproducibility. For this reason, most clinicians will treat all patients with advanced Hodgkin lymphoma with the same initial approach, regardless of the baseline characteristics. In the United Kingdom and the United States, this has generally meant using ABVD, whereas clinicians in Northern Europe have tended to favor eBEACOPP. The patient described in this case commenced treatment with ABVD.
Can therapy be adapted according to response?
Although baseline characteristics have been of limited usefulness in selecting therapy, there is increasing evidence that using functional imaging to make an early assessment of the response can be helpful, particularly for the likelihood of treatment failure, which may be amenable to correction if detected early. Hodgkin lymphoma shows avidity for FDG in almost all cases, and FDG-PET is an effective means of determining the metabolic activity in sites of involvement.39 Retrospective studies of patients treated with ABVD suggested that FDG-PET performed after 2 cycles of treatment can be highly predictive of treatment success or failure.40 This appears to provide better prognostic information than CT scans,41 with a high negative predictive value, giving a 2-year PFS of approximately 95% and a reasonable positive predictive value, with PFS between 13% and 27%.39 Interestingly, this assessment supersedes all the clinical baseline prognostic information.42 Thus, even patients with adverse baseline characteristics had an excellent prognosis if the interim FDG-PET was negative, whereas those with low initial risk showed poor PFS if the interim scan showed persistent metabolic activity. It is not clear however, whether the same observations hold true for more intensive regimens such as eBEACOPP.
In making these assessments, it is important to note that there are key issues of quality control in the performance of the scans and their interpretation, but the introduction of the 5-point scale for reporting has greatly improved the reliability and reproducibility of the results (Table 1).43,44 At interim, a score of 1 to 2 should be considered a complete metabolic response in patients receiving standard treatment; a score of 4 to 5 is considered positive and thus treatment has failed.39 A score of 3 is variably interpreted. In trials in which PET is used as a determinant of de-escalation, a score of 3 may be considered positive to prevent undertreatment. However, when interim scanning has been used to determine whether treatment should be escalated, then 3 is usually considered negative.
A number of prospective studies have now been performed that use interim FDG-PET assessment to guide the modulation of therapy. The international Response Adjusted Therapy for Hodgkin Lymphoma (RATHL) study (NCT00678327) tested the use of FDG-PET after 2 initial cycles of ABVD in more than 1200 patients, after which those with an interim PET score of 1 to 3 were randomly assigned to either continue ABVD or receive doxorubicin, vinblastine, and dacarbazine (AVD) without bleomycin to determine whether pulmonary toxicity could be reduced for those with a good prognosis.45 Conversely, patients with an interim PET score of 4 or 5 proceeded to intensification with either eBEACOPP every 3 weeks or the similar BEACOPP-14, given at 2-week intervals. The smaller US Intergroup study (NCT00822120) and the Italian lymphoma group study (NCT00795613) used a similar approach, with interim FDG-PET after 2 cycles of ABVD followed by intensification to BEACOPP for those with positive scans. The rates of metabolic remission after 2 cycles of ABVD were very similar in all 3 trials at about 85%.46-48 The results of treatment in the PET-positive group were also similar and appear to be superior to the historical controls used in previous studies, with subsequent metabolic response rates of 75% or more and projected failure-free survival figures at 2 years of 65% to 75%.
The importance of these findings is that they suggest that it may be possible to start with the less toxic ABVD and expose only the minority of patients for whom this is unlikely to be curative to the more intensive BEACOPP regimen. This will need to be confirmed by longer follow-up of the studies, but many centers, our own included, now carry out an interim FDG-PET scan after 2 cycles of ABVD and will intensify treatment on the basis of a persistently positive result. This was the case for the patient described, whose interim PET scan showed persistent (grade 4) FDG uptake in the left cervical nodes, with lower-level uptake in the mediastinal, paratracheal, and subcarinal nodes (Figure 1B). He went on to receive 4 cycles of BEACOPP-14 after which the scan became negative, and his treatment was completed with 2 further cycles. He has remained in remission since the completion of BEACOPP-14, 4 years ago.
At completion of chemotherapy
In those with a negative interim PET scan, we perform a restaging CT scan at the end of treatment. In those with a positive interim scan, we perform a further PET-CT scan to determine whether or not escalation of therapy has yielded a metabolic response. Biopsy of any persistently FDG-avid area should be carried out if possible, prior to commencing salvage chemotherapy. If only localized FDG avidity at a site of previous disease is present, then consolidation radiotherapy can be considered as an alternative.
Radiotherapy was the earliest form of effective treatment for Hodgkin lymphoma,49 but it has become an increasingly controversial component of treatment algorithms. This is mainly because of the emergent risks of long-term toxicity from second malignancies and accelerated cardiovascular disease within the irradiated field.50-56 Many of these problems arose from the use of extended fields and relatively high doses of radiation that were previously recommended; modern radiotherapy techniques appear to be much safer because both field size and dose are limited.57,58 Nonetheless, we recognize that it is preferable to avoid irradiation unless it adds significantly to the rate of cure. In advanced Hodgkin lymphoma, consolidation radiotherapy has conventionally been used for patients with a bulky site of disease at presentation or a residual mass at the completion of initial chemotherapy. Prior to the advent of functional imaging, a randomized trial showed that in patients treated with the hybrid MOPP-ABV (doxorubicin, bleomycin, vinblastine) regimen for 8 cycles, there was no advantage to using radiotherapy for those in complete radiologic remission.59 There appears to be a trade-off between the efficacy of prior chemotherapy and the advantage from irradiation: the more intensive the chemotherapy and the higher the complete remission rate, the less radiation adds to the results. In a large United Kingdom study, the elective omission of consolidation radiotherapy after ABVD was associated with inferior survival, despite the irradiated group’s having more bulky disease and fewer complete responses (CRs), indicating that conventional imaging is probably inadequate for selecting those in need of more treatment.60 Using FDG-PET to select residual masses that need consolidation appears to be a much better strategy. In the GHSG HD15 study, only patients with FDG-avid residual masses of more than 2.5 cm after BEACOPP therapy were selected for 30 Gy involved-field radiotherapy, with excellent results.61 Those with a PET-negative partial response had PFS of 92.1% at 4 years, very similar to those with a CR (92.6%), whereas even those who were PET-positive had 86.2% PFS. The use of radiotherapy in this study included only 11% of patients without any apparent reduction in survival by comparison with previous GHSG studies in which it was used in up to 70% of cases.
There are less conclusive data on the omission of radiotherapy in PET-negative patients after treatment with ABVD, but longer follow-up from the RATHL study45 should provide more clarity on this front. In many centers, consolidation radiotherapy is now reserved for those with a localized residual FDG-avid mass at the completion of chemotherapy.
What new agents hold significant promise?
After decades in which no new active agents emerged for treating Hodgkin lymphoma, the last few years have seen significant progress, in particular with the development of the antibody-drug conjugate brentuximab vedotin. This combines an antibody to the CD30 molecule, which is expressed on Reed-Sternberg cells, with an anti-tubulin, monomethyl auristatin E.62 The efficacy seen in early-phase studies has been impressive, with 76 objective responses (and 34% CRs) among 102 patients with recurrent disease who had already undergone high-dose therapy and autologous stem cell rescue.63 This level of efficacy as a single agent, combined with a favorable side effect profile largely confined to myelosuppression and neuropathy, has led to the investigation of brentuximab vedotin in combination with chemotherapy with a view to its incorporation into first-line treatment. Unexpected and severe pulmonary toxicity occurred when it was combined with ABVD,64 but the omission of bleomycin appears to have solved this problem, and a prospective trial, ECHELON-1 (NCT01712490) is now in progress testing AVD with brentuximab vedotin against ABVD in patients with stage III and IV disease. Entry to this trial would be my current preferred approach for a patient with advanced disease.
Blockade of the programmed death-1 (PD-1) pathway also holds significant promise. Pembrolizumab and nivolumab are two anti-PD-1 antibodies that have undergone phase 1 trials, with reported response rates of 53%65 and 87%,66 respectively. Nivolumab is currently being assessed in a phase 2 trial in patients whose disease has progressed after autologous stem cell transplantation (NCT02181738). A number of other new treatments are emerging, such as inhibitors of histone deacetylase,67 phosphoinositol 3-kinase,68 or the mammalian target of rapamycin,69 but these are at an earlier stage of investigation, and the results to date are less compelling.
How does our approach change for older patients?
Although the results of treatment of advanced Hodgkin lymphoma have improved markedly among those presenting in childhood, youth, or middle age, the outcomes in the 20% of patients older than 65 years at the time of diagnosis have changed much less.70 The prolonged use of bleomycin in ABVD in this group is attended by significant rates of severe pulmonary impairment (43% in one North American intergroup study71 ), and more intensive approaches such as BEACOPP result in high treatment-related mortality (21% in a GHSG trial72 ), a problem only partly attenuated by the omission of etoposide in the BEACOPP regimen, in which there were still 12% treatment-related deaths.73
Attempts have been made to develop regimens specifically for use in older patients, such as vinblastine, cyclophosphamide, prednisolone, procarbazine, etoposide, mitoxantrone, and bleomycin (VEPEM-B),74 which has been tested in a prospective United Kingdom study of 31 early-stage and 72 advanced-stage patients with a median age of 73 years.75 The 3-year PFS was 74% for early-stage patients and 58% for advanced-stage patients, with a low (1%) incidence of bleomycin toxicity. Similar results were seen with the GHSG prednisolone, vinblastine, doxorubicin, and gemcitabine (PVAG) regimen in a phase 2 study.76 Among 59 patients with a median age of 68 years, of whom 55 had advanced disease, 1 patient died of treatment-related toxicity, and the grade 3 to 4 respiratory toxicity rate was 7%. Three-year OS and PFS rates were 66% and 58%, respectively. The Nordic Lymphoma Group has investigated the cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) regimen and reported a retrospective series of 27 patients of median age 71 years (range, 61 to 80 years) of whom more than half had significant comorbidities.77 Among 18 patients with advanced disease, the CR rate was 72% and the 3-year PFS was 72%, with 1 treatment-related death.
Those who are older than age 65 years or who have abnormal pulmonary function tests should receive bleomycin for 2 cycles only or have it omitted altogether. In those with proven cardiac dysfunction or with cardiac risk factors, a nonanthracycline regimen such as chlorambucil, vinblastine, procarbazine, and prednisone (ChIVPP)78 may be considered. The dose of mediastinal radiotherapy should also be carefully considered.
In practice, it remains extremely difficult to specify the standard approach to treatment in older patients who constitute a heterogeneous group. Selection of a regimen depends on the severity and pattern of comorbidity, and it is clear that new approaches are needed. Several trials are in progress testing the use of brentuximab vedotin in this context, either as a single agent (ISRCTN77650947) or in combination with chemotherapy (NCT01476410 and NCT01716806) or rituximab (NCT01805037), and it is hoped that these may improve the benefit to toxicity ratio.
How do we follow up patients in first remission?
There is no substitute for taking a good history and performing a thorough clinical examination in the follow-up of Hodgkin lymphoma. Moreover, there is increasing evidence that patient-reported symptoms are the most effective means of detecting a recurrence.79 Follow-up frequency in trials has been based on the Cotswolds recommendations and consists of visits every 3 months during the first 2 years, every 4 months during the third year, and every 6 months during the fourth and fifth years.80 Chest x-rays are performed in those with previous mediastinal disease. We aim to discharge patients from routine follow-up at 5 years, with specific advice concerning monitoring for long-term toxicity or recurrence. Retrospective analysis has indicated that routine PET or CT scanning holds little value in identifying relapses81,82 and cannot be recommended in patients achieving a negative interim or end-of-treatment PET-CT scan.
The approach for refractory and recurrent disease
A range of salvage therapies have been tested in the 20% to 30%83 of patients with advanced disease whose lymphoma is refractory to, or relapses after, initial treatment. The salvage regimens appear to be largely interchangeable. The single-arm phase 2 trials performed do not offer direct comparison between regimens, but overall response rates of 60% to 80% have been found.84,85 A randomized comparison of sequential single-agent high-dose therapy versus continued conventional salvage therapy showed no difference in efficacy.86 Favored salvage regimens in our center include ifosfamide, carboplatin and etoposide (ICE), epirubicin in place of carboplatin (IVE), and cytarabine and cisplatin (DHAP).
Patients who are eligible and achieve a complete metabolic response should proceed to autologous stem cell transplantation in second remission. Assuming successful mobilization of stem cells, the standard high-dose regimen used prior to stem cell transplantation is carmustine, etoposide, cytarabine, and melphalan (BEAM). This is based on the 2 randomized controlled trials that compared high-dose chemotherapy followed by autologous stem cell transplantation to conventional chemotherapy in patients in whom a second remission had been achieved. Both studies showed significantly improved freedom from progression in the group receiving high-dose therapy, although the small numbers enrolled prevented conclusive demonstration of improved OS.87,88
Allogeneic transplantation may also have a role in the treatment of relapsed and refractory disease, especially for patients who suffer a further recurrence after autologous transplantation. Long-term survival benefit has not been demonstrated because of the absence of randomized trials and the potential for selection bias in cohort studies. In 1 prospective study of 78 patients with multiply-relapsed disease, 4-year estimated PFS and OS were 24% and 43%, respectively.89 A clinically significant graft-versus-lymphoma effect after donor lymphocyte infusion has been shown,90 and with improvement in treatment-related mortality with reduced-intensity regimens, this strategy remains an important option for those with poor responses to salvage therapy.
Conclusion
The modern treatment of advanced Hodgkin lymphoma is highly effective in the majority of patients, and much current research is directed at improving the subsequent quality of life while retaining the efficacy of first-line treatment. The key developments in this area are the increasing use of FDG-PET interim scanning to guide further treatment, and the introduction of new agents such as the antibody-drug conjugates, which appear to be one means to deliver a locally concentrated cytotoxic agent while avoiding high systemic exposure. The emergent data on immunomodulating antibody therapy is also encouraging, and a new generation of trials incorporating this approach is imminent.
Authorship
Contribution: P.J. and H.M. co-wrote the manuscript.
Conflict-of-interest disclosure: P.J. has received honoraria for participation in advisory boards and educational symposia from Millennium-Takeda and Bristol-Myers Squibb. The remaining author declares no competing financial interests.
Correspondence: Peter Johnson, Somers Cancer Research Building, Southampton General Hospital, Tremona Rd, Southampton SO16 6YD, United Kingdom; e-mail: johnsonp@soton.ac.uk.