In this issue of Blood, Duvic et al demonstrate that mogamulizumab, a humanized monoclonal antibody targeting the chemokine receptor CCR4, is well tolerated and has significant clinical activity (overall response rate 36.8%, median duration of response 10.4 months) in heavily pretreated patients with mycosis fungoides (MF) and Sézary syndrome (SS).1
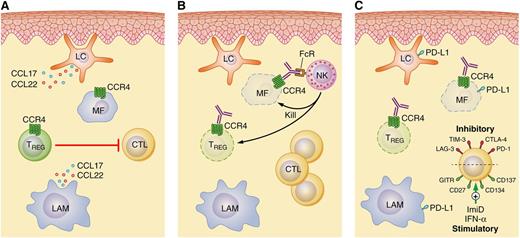
CCR4 as a therapeutic target in cutaneous T-cell lymphomas. (A) Langerhans cells (LC), LAM, and reactive T cells, including Treg cells that suppress reactive T helper and cytotoxic T cells (CTL), are abundant constituents of the tumor microenvironment in MF/SS. Interactions between the chemokines CCL17 and CCL22 and their receptor CCR4 facilitate the intricate crosstalk between a malignant T cell (MF cell) and its microenvironment. (B) Mogamulizumab triggers natural killer (NK) cell-mediated ADCC and depletion of CCR4+ MF and Treg cells. (C) Therefore, mogamulizumab may be rationally combined with immunomodulatory agents, both old (eg, interferons) and new (eg, with a monoclonal antibody targeting T-cell costimulatory or coinhibitory receptors), in future therapeutic strategies. IFN-α, interferon-α. Professional illustration by Patrick Lane, ScEYEnce Studios.
CCR4 as a therapeutic target in cutaneous T-cell lymphomas. (A) Langerhans cells (LC), LAM, and reactive T cells, including Treg cells that suppress reactive T helper and cytotoxic T cells (CTL), are abundant constituents of the tumor microenvironment in MF/SS. Interactions between the chemokines CCL17 and CCL22 and their receptor CCR4 facilitate the intricate crosstalk between a malignant T cell (MF cell) and its microenvironment. (B) Mogamulizumab triggers natural killer (NK) cell-mediated ADCC and depletion of CCR4+ MF and Treg cells. (C) Therefore, mogamulizumab may be rationally combined with immunomodulatory agents, both old (eg, interferons) and new (eg, with a monoclonal antibody targeting T-cell costimulatory or coinhibitory receptors), in future therapeutic strategies. IFN-α, interferon-α. Professional illustration by Patrick Lane, ScEYEnce Studios.
Limited-stage MF refractory to skin-directed therapies and advanced stage MF/SS are incurable with currently available therapies, with the exception of allogeneic transplantation. Conventional chemotherapeutic agents have clinical activity in these patients, but durable remissions are rarely achieved. Among patients initiating chemotherapy, a disappointing median time-to-next treatment of <4 months is anticipated, and ∼90% will require an alternative therapy within 1 year of treatment initiation.2 Responses with biological response modifiers and histone deacetylase inhibitors, although superior to conventional chemotherapy, are frequently short-lived; most patients will experience disease relapse/progression within 1 year of treatment initiation. Given its mechanism(s) of action, tolerability, and ability to be rationally combined with other novel agents, the CCR4-targeting monoclonal antibody mogamulizumab is a welcome, and much needed, addition to the MF/SS arsenal.
Mogamulizumab targets CCR4, a chemokine receptor that is preferentially expressed by Th2 and regulatory T (Treg) cells. In response to its ligands, CCL17 (TARC) and CCL22 (MDC), CCR4 promotes T-cell migration to extranodal sites, including the skin. These CCR4 ligands, produced by keratinocytes, dendritic cells (including Langerhans cells), and macrophages, are abundant within MF/SS-involved skin (see figure). A recently described subset of peripheral T-cell lymphomas expresses CCR4 as part of the transcriptional repertoire of GATA-3, the “master” transcriptional regulator driving Th2 differentiation. GATA-3 is not only expressed by Treg cells residing within barrier sites such as the skin, but also by MF/SS cells, and may drive CCR4 expression in these cells.3,4 Furthermore, GATA-3–dependent cytokines produced by malignant T cells, particularly interleukin-4/interleukin-13, functionally polarize lymphoma-associated macrophages (LAMs). Polarized LAMs, in turn, produce CCL17 and CCL22, which interact with CCR4 on malignant T cells and facilitate tumor–microenvironment crosstalk. The CCL17/CCL22–CCR4 axis in MF/SS is further maintained by the absence of CD26, a dipeptydil peptidase that normally inactivates CCR4 ligands.
In addition to its role in regulating cell homing and trafficking, CCR4 engagement may also promote cell growth and survival. However, cells can become desensitized to CCR4 through receptor internalization, a homeostatic regulatory mechanism. Gain-of-function mutations in the CCR4 cytoplasmic domain inhibit CCR4 internalization and promote phosphatidylinositol 3-kinase/AKT activation. These mutations were recently described in ∼25% of adult T-cell leukemia/lymphomas.5 Clearly, CCR4 has a pathogenic role in MF/SS and other T-cell lymphoproliferative disorders and is an attractive therapeutic target.
Mogamulizumab depletes CCR4-expressing cells by antibody-dependent, cell-mediated cytotoxicity (ADCC). Defucosylation of its Fc region culminates in enhanced Fc receptor binding, permitting ADCC at lower antigen densities and at lower ratios of effector cells to target cells. Or, viewed in a different light, mogamulizumab completely cleared the peripheral blood of malignant T cells in >50% of patients at a concentration (1 mg/kg) that is approximately 1/10th that of rituximab (ie, 375 mg/m2 or ∼10 mg/kg). In addition to directly targeting malignant T cells expressing CCR4, mogamulizumab depletes Treg cells, an important therapeutic target in many human cancers because of their role in suppressing host antitumor immunity. In a recent companion study, Duvic and her colleagues observed a significant reduction in peripheral blood Treg cells following treatment with mogamulizumab.6 A similar reduction in Treg cells was also observed in a mogamulizumab-treated cohort of peripheral T-cell lymphoma patients.7 Therefore, in addition to directly targeting malignant T cells expressing CCR4, mogamulizumab may favorably influence the tumor microenvironment upon Treg depletion (see figure) without triggering clinically significant autoimmune complications. Given its dual mechanism of action, mogamulizumab may effectively “kill 2 birds with 1 stone” and may be rationally combined with other immunomodulatory agents in future studies. For example, immunomodulatory drugs and agonistic antibodies targeting T-cell costimulatory receptors, including CD137 (4-1BB),8,9 may further augment ADCC and T cell–mediated immunity. Because PD-L1 is frequently expressed in MF/SS,10 anti–PD-L1/PD-1 checkpoint blockade is a similarly attractive combinatorial approach. The clinically meaningful responses observed with mogamulizumab in a heavily pretreated cohort of MF/SS patients, and the possibility of future combinatorial strategies (see figure), suggest that we are witnessing the dawn of an exciting new era in MF/SS management.
Conflict-of-interest disclosure: The author declares no competing financial interests.