In this issue of Blood, Pasquier et al report on a randomized control trial demonstrating no important improvement in live birth rates in women with prior recurrent early pregnancy loss with antepartum low-molecular–weight heparin (LMWH) injections.1
Most women, and their partners, who experience pregnancy loss are devastated by these outcomes and desperately seek answers. They ask hematologists the etiologic question of whether inherited thrombophilias cause pregnancy loss and the therapeutic question of whether LMWH can prevent recurrent pregnancy loss.
Inherited thrombophilias are associated with an increased risk of recurrent early and late pregnancy loss, but for the most common thrombophilias, these associations are weak, suggesting that inherited thrombophilias are weak contributors rather than the sole cause of pregnancy loss.2 In other words, they are unlikely the etiologic answers these women and their partners seek.
LMWH has been explored to prevent pregnancy loss in women with and without thrombophilia with prior pregnancy loss, with disappointing results. However, these negative results are important because, unfortunately, many women have demanded and many clinicians have provided LMWH injections based on hope, rather than evidence, that LMWH can prevent recurrent pregnancy loss.
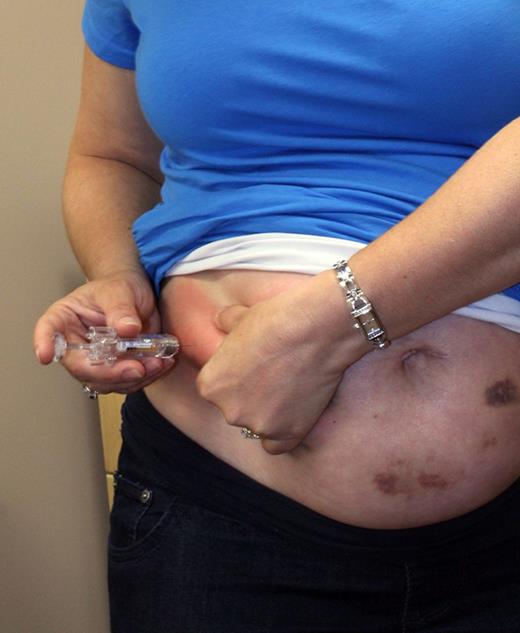
The off-label use of LMWH has been fueled by not only the emotional impact of pregnancy loss but also expert opinion, consensus panels,3 and small nonrandomized studies suggesting benefit. LMWH is not a benign intervention; it can be complicated by heparin-induced thrombocytopenia (albeit rarely), withholding of epidural analgesia, increased minor bleeding,4,5 allergic reactions,4 skin reactions,4 raised liver transaminase concentrations,5 and increased likelihood of induction of labor. Also, up to 400 subcutaneous LMWH injections per term pregnancy is both a personal (see figure) and financial burden (up to $15 000 per pregnancy).
Pasquier et al1 conducted a well-designed double-blind randomized controlled trial exploring the potential benefit of enoxaparin 40 mg vs placebo injections in women with prior recurrent pregnancy loss but no thrombophilia. Although the trial was stopped early for futility, it excludes an important treatment effect (10% improvement in live birth rate with enoxaparin). This trial is consistent overall with open-label trials in similar populations. Although some of these trials have suggested a benefit in reducing recurrent early6 and late pregnancy loss,7 other large trials, in general more methodologically sound, have suggested no benefit of LMWH in preventing recurrent early pregnancy loss.8-10 Overall, it is time to put the LMWH needles away and not offer this therapy to women with prior recurrent pregnancy loss in the absence of thrombophilia.
A similar story is emerging in women with prior recurrent pregnancy loss who have inherited thrombophilia. Three prior randomized trials have assessed the efficacy of LMWH in thrombophilic pregnant women with prior pregnancy loss. Gris et al compared enoxaparin 40 mg daily with aspirin in 160 women with inherited thrombophilia and 1 prior late pregnancy loss (>10 weeks of gestation).7 Enoxaparin resulted in a higher live birth rate (86%; 69 live births/80 pregnancies) than aspirin (29%; 23 live births/80 pregnancies; −57% absolute improvement in live birth rate). However, the unusually high rate of fetal loss after the eighth week (42.5%) compared with the 7% rate of fetal loss prior to the eighth week has led to skepticism about the external generalizability of this study’s findings. The Thrombophilia in Pregnancy Prophylaxis Study (TIPPS) trial explored the effect of prophylactic dose dalteparin compared with no dalteparin and did not show a benefit in the subgroups with recurrent early loss (n = 44) or those with prior late loss (n = 81), but these subgroup analyses are hypothesis generating at best.5 An ongoing randomized controlled trial will hopefully shed further light on the potential benefit of LMWH to prevent recurrent pregnancy loss in thrombophilic women (www.trialregister.nl #NTR 3361). The bottom line is that we need further research to explore whether LMWH can improve live birth rates in women with thrombophilia. However, until this research is completed, LMWH should not be offered to women outside of clinical trials based on the weak association highlighted above and the lack of good-quality evidence to support benefit.
Conflict-of-interest disclosure: The author declares no competing financial interests.