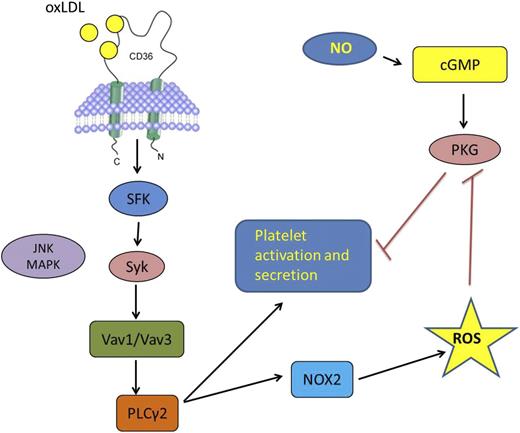
In this issue of Blood, Magwenzi et al from the University of Hull report a novel mechanistic connection between oxidized low-density lipoprotein (oxLDL)-induced prothrombotic platelet signaling and the inhibition of endogenous platelet anti-activating signaling mediated by the nitric oxide (NO)/guanosine 3′,5′-cyclic monophosphate (cGMP)/protein kinase G (PKG) pathway.1
The oxLDL interaction with CD36 on the surface of a platelet triggers a signaling cascade involving specific SFK, syk, MAPK c-Jun N-terminal kinase, Vav-family guanine nucleotide exchange factors, and PLCγ2, leading ultimately to the activation of the NOX2 isoform of NADPH oxidase and generation in intracellular ROS, including superoxide ion. This pathway leads directly to platelet activation and secretion, and the studies by Magwenzi et al showed that ROS also contribute to platelet activation by inhibiting the NO/cGMP/PKG pathway that normally serves as an inhibitor or “brake” on platelet activation.
The oxLDL interaction with CD36 on the surface of a platelet triggers a signaling cascade involving specific SFK, syk, MAPK c-Jun N-terminal kinase, Vav-family guanine nucleotide exchange factors, and PLCγ2, leading ultimately to the activation of the NOX2 isoform of NADPH oxidase and generation in intracellular ROS, including superoxide ion. This pathway leads directly to platelet activation and secretion, and the studies by Magwenzi et al showed that ROS also contribute to platelet activation by inhibiting the NO/cGMP/PKG pathway that normally serves as an inhibitor or “brake” on platelet activation.
Platelets are activated in response to vascular injury via specific signaling receptors, including G–protein coupled receptors for thrombin and adenosine 5′-diphosphate (ADP) and adhesion receptors such as GPIb complex, integrins, and glycoprotein VI. Activation is regulated, however, by intracellular inhibitory pathways triggered by soluble mediators, such as prostacyclin and NO, or by clustering the immunoreceptor tyrosine-based inhibitory motif containing transmembrane protein platelet endothelial cell adhesion molecule. Disabling the platelet’s natural “braking” system would be predicted to enhance activation and thus promote thrombosis, and indeed Magwenzi et al showed that the prothrombotic activity of oxLDL was mediated, at least in part, by CD36-induced generation of intracellular reactive oxygen species (ROS) via the reduced NAD phosphate oxidase 2 (NOX2) isoform of reduced NAD phosphate (NADPH) oxidase. The investigators convincingly used a combination of pharmacologic and biochemical approaches, along with mouse genetic models and ex vivo assays of platelet function to identify key molecular components of the pathway and show potential relevance to arterial thrombosis.
For many years, studies have hinted that circulating platelets in patients with common diseases, such as diabetes, hyperlipidemia, atherosclerosis, cancer, and chronic systemic vasculitis are “hyperreactive,” and this may relate to the elevated risk of myocardial infarction and stroke associated with these conditions. More recently, some of the molecular underpinnings of this platelet hyperreactivity have been identified. Platelets express on their surface several receptors known to be components of the innate immune system, including toll-like receptors and scavenger receptors, and thus continuously scan the intravascular environment for the presence of endogenous and exogenous danger signals, ie, the so-called danger-associated molecular patterns (DAMPs). The type 2 scavenger receptor CD36, originally identified as platelet glycoprotein IV, is densely expressed on the platelet surface and serves as a recognition site for several DAMPs that are highly relevant to human diseases, including oxLDL,2 advanced glycated proteins,3 cell-derived microparticles,4 the pro-inflammatory peptide S100A9,5 malaria-infected erythrocytes, and liposaccharide structures expressed on pathogenic bacteria, including staph and mycobacteria.6 Platelet interaction with these danger signals via CD36 initiates a signaling cascade that promotes platelet activation and thrombus generation. Presumably, platelet recognition of foreign invaders or endogenous molecules and cells modified by oxidant stress and other forms of injury offers an evolutionary advantage, perhaps by generating a “mini-thrombus” that sequesters the invader and facilitates clearance. Unfortunately, this system may also promote pathological platelet activation leading to arterial occlusion. Our laboratory and others have begun to identify the signaling cascades in platelets triggered by CD36, showing that activation of specific src-family kinases (SFK) (fyn and lyn), mitogen-activated protein kinases (MAPK), phospholipase C and Syk, and Vav-family guanine nucleotide exchange factors6-8 are required for direct platelet activation by oxLDL and other DAMPs (see figure, left side) and that targeting these signaling molecules in vivo in mice can ameliorate the prothrombotic state associated with diabetes, hyperlipidemia, and oxidant stress. The work by Magwenzi et al1 extends this story in important new directions by showing that oxLDL-triggered CD36 signaling also promotes thrombosis by disabling the natural platelet regulatory pathways that blunt activation (see figure, right side). This may explain the observations that CD36 deficiency shifts the dose response of platelets to classic agonists to the right, such as ADP4 and collagen, and that CD36 ligands in normal platelets increase platelet reactivity to low doses of these agonists (ie, shifts the dose response to the left). In addition, although it has been shown that some components of the CD36 signaling pathway in other cell systems, including macrophages, vascular endothelial cells, and vascular smooth muscle cells require generation of intracellular ROS,6,9 this is the first demonstration of CD36-mediated ROS signaling in platelets, and the first to connect ROS generation to blunting of natural platelet inhibitory pathways. Many important questions remain to be addressed, including the specificity of CD36 signaling, ie, whether CD36 DAMP ligands other than oxLDL trigger the same response to generate intracellular ROS. Recent studies have shown that CD36 signaling in macrophages is quite complex and often requires cooperation with other membrane proteins, including toll-like receptors,10 tetraspanins, integrins, and the sodium-potassium ATPase. Whether these CD36 partners are involved in ROS generation and whether different DAMPs generate differential downstream signals based on their capacity to recruit specific CD36 membrane partners remains to be determined, as do the mechanisms by which ROS target the cGMP signaling pathway. This interesting paper, however, points to potential new targets for lowering thrombotic risk in highly susceptible patient populations.
Conflict-of-interest disclosure: The author declares no competing financial interests.