In this issue of Blood, Dertschnig et al describe the development of autoreactive T cells from the thymus in mice that had previously developed acute graft-versus-host-disease (aGVHD).1
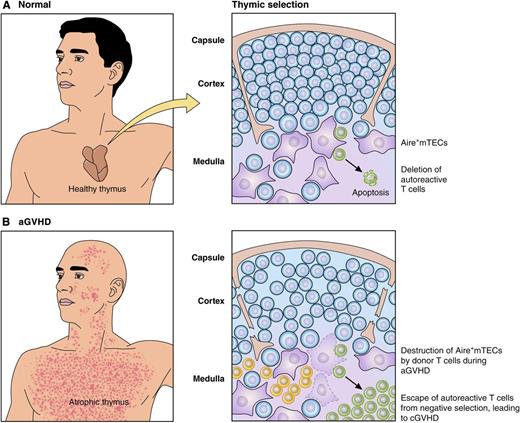
Alloreactive donor T cells destroy Aire+mTECs during aGVHD, allowing escape of autoreactive T cells that contribute to cGVHD. (A) In healthy individuals, mTECs (purple) mediate negative selection of thymocytes (blue) to eliminate autoreactive T cells (green) recognizing self-peptides presented on MHC molecules. (B) During aGVHD, alloreactive donor T cells (yellow) damage the thymus and eliminate mTECs, allowing escape of autoreactive T cells (green) that contribute to the pathogenesis of cGVHD in the periphery. Professional illustration by Patrick Lane, ScEYEnce Studios.
Alloreactive donor T cells destroy Aire+mTECs during aGVHD, allowing escape of autoreactive T cells that contribute to cGVHD. (A) In healthy individuals, mTECs (purple) mediate negative selection of thymocytes (blue) to eliminate autoreactive T cells (green) recognizing self-peptides presented on MHC molecules. (B) During aGVHD, alloreactive donor T cells (yellow) damage the thymus and eliminate mTECs, allowing escape of autoreactive T cells (green) that contribute to the pathogenesis of cGVHD in the periphery. Professional illustration by Patrick Lane, ScEYEnce Studios.
The findings of Dertschnig et al provide an important mechanistic link between the pathogenesis of acute graft-versus-host disease (aGVHD) and its more indolent cousin, chronic (c)GVHD.2,3 aGVHD typically occurs in the first 100 days after allogeneic hematopoietic stem cell transplant (HSCT), and is mediated by mature T cells present in the donor graft that cause local inflammation and damage epithelial cells in the skin, liver, and gastrointestinal tract.2 In contrast, cGVHD typically develops 4 to 6 months posttransplant due to antigen-specific donor immune cells that cause autoimmune clinical manifestations including sclerosis and fibrosis in tissues and organs.3 After donor stem cells have engrafted in the bone marrow, including the setting of major histocompatibility complex (MHC) mismatched donor and recipient,4 donor-derived T cells developing in the recipient thymus should undergo negative selection, one aspect of central tolerance, to eliminate autoreactive clones. Thus, the presence of autoreactive donor-derived T cells in the periphery that recognize self-peptides in patients with cGVHD represents a failure of negative selection. Although aGVHD is well established as a risk factor for the development of cGVHD,5 the mechanism for the association has not been clear. A clue to understanding the relationship between acute and chronic GVHD is based on the normal process by which autoreactive T cells are eliminated. Activity of the Aire gene in the thymus leads to low levels of synthesis of a smorgasbord of tissue-restricted proteins and subsequent presentation of peptides derived from these proteins on medullary thymic epithelial cells (mTECs). During physiological negative selection, thymocytes that are autoreactive to proteins expressed in peripheral organs are eliminated when they come into contact with mTECs expressing peptides normally restricted to peripheral tissues. The study by Dertschnig et al provides important insight into how negative selection fails in the setting of allogeneic transplant, and connects the pathophysiology of aGVHD to the subsequent development of cGVHD.1
The authors use a RIP-mOVA mouse model system, in which ovalbumin (OVA) is expressed under the control of the tissue-specific rat insulin promoter (RIP), as a model for a tissue-specific protein that should cause negative selection in the thymus. Using RIP-OVA transgenic mice as transplant recipients, membrane-bound (m)OVA is expressed in pancreatic islets and by Aire+mTECs, normally leading to thymic elimination of autoreactive T cells that recognize mOVA peptides. The authors established that alloreactive T cells that are present in a donor graft from an MHC-mismatched mouse strain cause destruction and elimination of Aire+mTECs during aGVHD. Mice that had developed aGVHD and lacked Aire+mTECs were then retransplanted with congenic T cell–depleted bone marrow from MHC matched OT-II donor mice that express a T-cell receptor on CD4+ T cells specific to an mOVA peptide. The authors show that when negative selection is intact in control mice that did not develop aGVHD, transgenic OT-II T cells are deleted from the repertoire during intrathymic T-cell development. In mice with a history of aGVHD and that lack Aire+mTECs expressing mOVA, OT-II T cells survived negative selection and migrated to the periphery unchecked. The findings of Dertschnig et al illustrate the relationship between aGVHD and the failure of central tolerance: autoreactive T cells were generated de novo following the second MHC-matched transplant of T cell–depleted OT-II bone marrow due to loss of mTECs in the damaged thymus (see figure).
Dertschnig et al show that the mOVA-specific T cells generated in their system are highly reactive to mOVA peptide in culture but leave unanswered the important question of whether these mOVA-specific T cells are generated in sufficient number and with the functional ability to cause autoimmunity in these mice. Because mOVA is under the RIP promoter in the transgenic mice used in this model, this question could be answered by assessing damage to pancreatic islet cells and ensuing Type 1 diabetes. Findings pertaining to this question have the potential to further the understanding of the link between cGVHD and the graft-versus-leukemia (GVL) effect of the allogeneic transplant. Because the development of cGVHD is also associated with a reduced risk of leukemia relapse and increased GVL activity, it may be that leukemia-associated antigens are also ectopically expressed by Aire+mTECs. If so, elimination of mTECs may permit the survival of GVL-specific donor-derived T cells generated de novo in the recipient thymus. However, the contribution of the thymus to the GVL effect may be limited by decreased thymic T-cell output due to atrophy and depopulation and thinning of the thymic cortex that follows the development of aGVHD (see figure).6
With the findings from Dertschnig et al in hand, what methods can be used to eliminate, reduce, or alter function of alloreactive T cells in donor hematopoietic stem cell grafts? Although outright elimination of all alloreactive effector T cells in allogeneic HSCT has had limited success due to increased risks of leukemia relapse and delayed immune reconstitution, a promising new approach is to use recipient cells to condition donor grafts to generate antigen-specific regulatory T (Treg) cells that limit GVHD and tissue damage when transplanted in combination with alloreactive T cells.7 Such an approach might be of great value if the immune-dominant peptides that are the targets of donor T cells that mediate cGVHD attack of the skin, liver, and lungs might be used to generate Treg cells that limit cGVHD while sparing cytotoxic effector cells that mediate GVL. It is also of interest to determine whether ex vivo–generated donor Treg cells could be used to prevent the damage to the thymus during aGVHD and reduce the incidence of subsequent cGVHD.
Dertschnig et al eloquently demonstrate that destruction of Aire+mTECs during aGVHD leads to de novo generation of inappropriately licensed autoreactive T cells and has helped “clear the Aire” regarding the pathophysiology of acute and chronic GVHD.
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.