To the editor:
Familial hemophagocytic lymphohistiocytosis (FHL), a rare autosomal recessive disorder of lymphocyte cytotoxicity, is caused by mutations in genes encoding perforin (FHL-2) or proteins important for intracellular trafficking/exocytosis of perforin-containing lytic granules: Munc13-4 (FHL-3), syntaxin 11 (FHL-4), and Munc18-2 (FHL-5).1 FHL-1 is due to an unidentified gene defect located on chromosome 9. Munc13-4 (a Rab27a effector) coordinates exocytosis in hematopoietic cells.2,3 Munc13-4 deficiency (FHL-3) results in defective cytolytic granule exocytosis.4 Interestingly, platelets from Munc13-4–deficient mice showed a severe secretion defect of α- and dense granules.5 In patients with FHL-3, bleeding symptoms have been rarely reported because patients may have not been challenged (ie, surgery).6 An 8-month-old Chinese FHL-3 patient died because of gastrointestinal hemorrhage.7 A platelet secretion defect in patients with FHL-5 has been described previously.8,9
Therefore, we analyzed platelet function in 2 unrelated male patients with genetically confirmed FHL-3. Both patients presented with clinical symptoms indicative of hemophagocytic lymphohistiocytosis (HLH) and fulfilled the diagnostic criteria.10 Functional analyses showed absent degranulation of cytotoxic T cells and reduced degranulation of natural killer cells. Patient 1 (diagnosed at the age of 4 months) showed compound heterozygous mutations in the UNC13D gene accounting for FHL-3: c.551G>A (p.W184X) in exon 6 and c.118-308C>T in intron 1. After treatment according to the HLH 2004 protocol, haploidentical hematopoietic stem cell transplantation was performed at the age of 16 months.10 Patient 2 (diagnosed at the age of 5 months) had severe central nervous system involvement and showed compound heterozygous splice donor site mutations of UNC13D: c.753+1G>T of exon 9 and c.1389+1G>A of exon 15. The patient received treatment according to the HLH 2004 protocol and underwent hematopoietic stem cell transplantation from a matched unrelated donor at the age of 9 months. He died of HLH relapse due to graft failure 4 months later. No major bleeding episodes were observed in either patient. The platelet studies were performed shortly before transplantation when patients were stable and did not receive medication, which induced secretion defects. Platelet count was normal at the time of platelet studies.
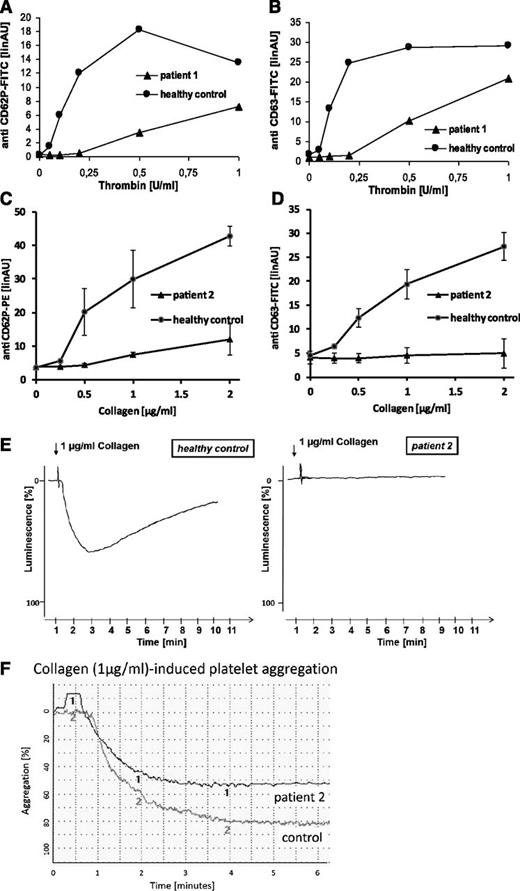
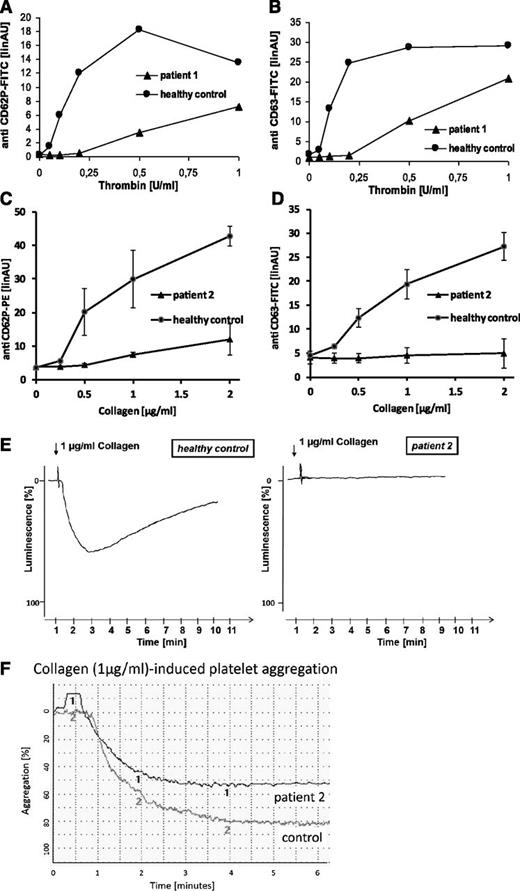
Flow cytometric analyses of platelets from both patients revealed severely diminished to absent platelet α- (CD62P) and dense granule (CD63) secretion in response to thrombin, collagen, and thrombin receptor activating peptide (TRAP) in platelet-rich plasma (Figure 1A-D; TRAP not shown). Collagen-induced adenosine triphosphate secretion (lumiaggregometry) in whole blood was absent (Figure 1E), whereas agonist-induced fibrinogen binding (data not shown) and aggregation (Figure 1F) were normal for a child of that age. In addition, flow cytometric analyses of platelets from patient 2 showed absent mepacrine uptake and release (a marker of uptake and release of dense body contents), as well as absent expression of the lysosomal marker CD107a (LAMP-1) in response to collagen and TRAP (data not shown).
Platelet function analyses. Flow cytometric quantification of platelet granule secretion in platelet-rich plasma was stimulated using increasing concentrations of thrombin (0, 0.05, 0.1, 0.2, 0.5, and 1.0 U/ml) (A-B) or collagen (0, 0.25, 0.5, 1, and 2 µg/ml) (C-D). After fixation cells were washed and incubated with fluorescein isothiocyanate-conjugated anti-CD62 (A), phycoerythrin-conjugated anti-CD62 (C), or fluorescein isothiocyanate-conjugated anti-CD63 (B,D). Surface fluorescence was analyzed with a flow cytometer (FACSCalibur; Becton Dickinson). Data are expressed as linear arbitrary units. Analyses were performed with patients’ platelets and platelets from a healthy control. Platelet adenosine triphosphate release in response to collagen was monitored by lumiaggregometry in whole blood (E). Relative luminescence in percent was plotted as a function of time. Analyses were performed with platelets from patient 2 and platelets from a healthy control. Collagen-induced platelet aggregation of platelets from patient 2 and platelets from a healthy control was analyzed using a PAP4-aggregometer (F).
Platelet function analyses. Flow cytometric quantification of platelet granule secretion in platelet-rich plasma was stimulated using increasing concentrations of thrombin (0, 0.05, 0.1, 0.2, 0.5, and 1.0 U/ml) (A-B) or collagen (0, 0.25, 0.5, 1, and 2 µg/ml) (C-D). After fixation cells were washed and incubated with fluorescein isothiocyanate-conjugated anti-CD62 (A), phycoerythrin-conjugated anti-CD62 (C), or fluorescein isothiocyanate-conjugated anti-CD63 (B,D). Surface fluorescence was analyzed with a flow cytometer (FACSCalibur; Becton Dickinson). Data are expressed as linear arbitrary units. Analyses were performed with patients’ platelets and platelets from a healthy control. Platelet adenosine triphosphate release in response to collagen was monitored by lumiaggregometry in whole blood (E). Relative luminescence in percent was plotted as a function of time. Analyses were performed with platelets from patient 2 and platelets from a healthy control. Collagen-induced platelet aggregation of platelets from patient 2 and platelets from a healthy control was analyzed using a PAP4-aggregometer (F).
These data demonstrate a selective impairment of platelet granule secretion in patients with FHL-3, and thus support an important role for Munc13-4 in human platelet degranulation. Although bleeding symptoms in FHL-3 patients can be mild, our findings clearly demonstrate that Munc13-4 deficiency is more than a genetic disorder of cytotoxicity.
Authorship
Contribution: A.B., M.F.B., S.A., and L.N. performed research and analyzed data; B.E.K., B.Z., and K.B. designed research, analyzed data, and wrote the paper; K.S.-L. analyzed data and wrote the paper; U.z.S. performed genetic analysis; and K.B. and A.S.S. took care of the patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara Zieger, University Hospital Freiburg, Department of Pediatrics and Adolescent Medicine, Mathildenstrasse 1, 79106 Freiburg, Germany; e-mail: barbara.zieger@uniklinik-freiburg.de.
References
Author notes
L.N., A.B., B.Z., and B.E.K. contributed equally to this study.