In this issue of Blood, Laurent et al demonstrate that phosphatidylinositol 3-kinase β (PI3Kβ) activity is essential for thrombus stability at a high shear rate, highlighting a potential risk of embolization upon PI3Kβ inhibition.1
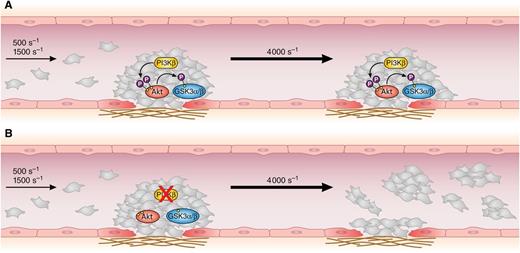
Inside a growing thrombus (A), platelet PI3Kβ promotes Akt activation and the subsequent phosphorylation and inhibition of GSK3α/β. These reactions stabilize and consolidate the thrombus, which becomes resistant even to a sudden elevation of the shear rate. (B) In the absence of platelet PI3Kβ, or upon inhibition of PI3Kβ activity, the thrombus grows normally at physiological shear rates, but Akt and GSK3α/β are not phosphorylated. This prevents thrombus stabilization, and the increase of the shear rate causes the detachment of platelet emboli, leaving a layer of collagen-adherent cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
Inside a growing thrombus (A), platelet PI3Kβ promotes Akt activation and the subsequent phosphorylation and inhibition of GSK3α/β. These reactions stabilize and consolidate the thrombus, which becomes resistant even to a sudden elevation of the shear rate. (B) In the absence of platelet PI3Kβ, or upon inhibition of PI3Kβ activity, the thrombus grows normally at physiological shear rates, but Akt and GSK3α/β are not phosphorylated. This prevents thrombus stabilization, and the increase of the shear rate causes the detachment of platelet emboli, leaving a layer of collagen-adherent cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
The stringent need to improve current antithrombotic therapies urges the identification of novel targets for antiplatelet treatments. The elucidation of the molecular mechanisms and critical players of platelet activation represents a mandatory approach to identify suitable intracellular effectors able to control specific signaling pathways. In the last 10 years, a growing volume of evidence has pointed to the β isoform of PI3Kβ as the most promising novel target for antithrombotic drugs,2 and PI3Kβ selective inhibitors have revealed a remarkable ability to prevent platelet activation and thrombus formation without affecting hemostasis.3 The study by Laurent et al1 documents a previously unrecognized function for platelet PI3Kβ in thrombus stabilization at a high shear rate and thus provides an important warning that therapeutic inhibition of PI3Kβ may increase the risk of embolization and secondary ischemic events in the microcirculation.
PI3Kβ belongs to the class I PI3K family, enzymes able to produce phosphatidylinositol 3,4,5-trisphosphate in the living cell.4 This membrane-anchored second messenger initiates intracellular signaling pathways by recruiting pleckstrin homology domain–containing proteins, including the protein kinases PDK1 and Akt. PDK1 phosphorylates Akt on Thr308, priming the subsequent phosphorylation on Ser473 required for full activation. Akt propagates PI3K-initiated signals by phosphorylating many downstream substrates, including glycogen synthase kinase 3 (GSK3), to initiate pathways whose precise contribution to platelet activation is still largely unclear.
Platelets express all the 4 different isoforms of class I PI3Ks (α, β, δ, and γ), but it is well documented that PI3Kβ plays a predominant role in the regulation of adhesive function. The availability of isoform-specific inhibitors and the development of selective transgenic mice allowed the recognition that PI3Kβ, but not other class I isoforms, is essential for platelet activation by collagen,5-7 is a critical regulator of integrin αIIbβ3 outside-in signaling,6-8 and contributes to platelet activation by G protein-coupled receptor stimulation.6,7 Inhibition of PI3Kβ or genetic ablation of its activity impaired platelet adhesion and spreading on collagen or fibrinogen, affected clot retraction, inhibited platelet aggregation, altered thrombus formation under flow, and prolonged the arterial occlusion time in different models of arterial thrombosis.6,7 According to this, a novel, potent and short-acting PI3Kβ inhibitor, AZD6482, has been proven to display straight antithrombotic effects without altering hemostasis and has been validated in animal models as well as in humans.3
The core observation of the study by Laurent et al1 is simple but striking (see figure): when blood from mice with selective deletion of p110β in the megakaryocyte lineage is perfused over a collagen matrix at physiological shear rates, thrombus formation and growth are comparable to those observed with control blood. However, a sudden acceleration of blood flow to a pathological shear rate of 4000 s−1 causes the rapid and almost complete detachment of platelets for the thrombus only in the absence of PI3Kβ. Accordingly, blood perfusion at 4000 s−1 generates thrombi of PI3Kβ-null platelets that start to grow normally, but when a critical size is reached, they become unstable and a progressive detachment of platelets occurs. Thus, PI3Kβ is not actually required for thrombus growth and stability at a physiological shear rate, but it is essential to preserve thrombus integrity when the shear rate is increased. Two additional important observations integrate these findings. First, thrombus instability at a high shear rate is also documented when human platelets are treated with the selective PI3Kβ inhibitor AZD6482. Second, monitoring thrombus formation in vivo by intravital microscopy upon laser-induced deep lesion of mesenteric arterioles reveals that the absence of platelet PI3Kβ significantly increases embolization with detachment of platelet aggregates from the largest thrombi.
Two questions arise from this study. What is the mechanism for PI3Kβ-mediated maintenance of thrombus stability at a high shear rate? Should this role of PI3Kβ be regarded as a serious concern when pursuing its inhibition for antithrombotic purposes?
The present study shows that the lack of PI3Kβ specifically weakens the platelet-platelet contact inside the thrombus rather than the platelet-matrix interaction. This points to a defective integrin αIIbβ3 function in the absence of PI3Kβ as the cause for thrombus instability, a hypothesis consistent with the documented role of PI3Kβ in integrin outside-in signaling.6-9 Defective avidity regulation of integrin αIIbβ3 may reduce the strength of cell-cell interaction to levels sufficient to support and consolidate large thrombi at a physiological shear rate but inadequate to resist to stronger hemodynamic forces. Laurent et al1 demonstrate that this effect is mediated by Akt-dependent phosphorylation of GSK3, a negative regulator of platelet function whose inhibition is promoted by Akt phosphorylation.10 The authors show that Akt and GSK3 are phosphorylated in the growing thrombus by a PI3Kβ-dependent mechanism and that, in the absence of PI3Kβ, pharmacologic inhibition of GSK3 efficiently restores the thrombus stability at a high shear rate. This observation represents an important piece of information, although it defers to future investigations the explanation of the mechanism for integrin regulation by GSK3.
The demonstration that PI3Kβ inhibition causes thrombus instability and increases the shedding of platelet emboli from large thrombi at a high shear rate may attenuate the enthusiasm toward this so-far promising antithrombotic target. Although thrombus instability in the absence of PI3Kβ is seen only at a pathological high shear rate, intravital microscopy analysis documented the real detachment of platelet thrombi from injured arterioles. The authors consider that the growth of a thrombus itself may alter the rheological conditions of the vessel and produce a local elevation of the shear rate, increasing embolization upon PI3Kβ inhibition. This argument strengthens the physiological relevance of the reported observations. Nevertheless, in vivo embolization is documented only in PI3Kβ-null mice, a model in which PI3Kβ activity is completely abrogated. In contrast, pharmacologic inhibitors may produce a partial reduction of the enzymatic activity of PI3Kβ in circulating platelets. Therefore, it now appears essential to determine whether a precise calibration of the dose of PI3Kβ inhibitors administered to animals may allow one to define conditions preserving the benefits of the antithrombotic effects while reducing the risk of increased embolization and possibly secondary ischemic events.
Conflict-of-interest disclosure: The author declares no competing financial interests.