In this issue of Blood, Nai et al establish a function of transferrin receptor 2 (TfR2) as a negative regulator of erythropoiesis.1
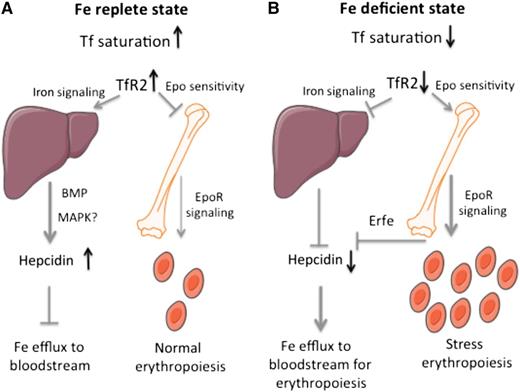
A model for the function of hepatic and erythroid TfR2 in the control of systemic iron metabolism and erythropoiesis. (A) In an iron-replete state, stabilized TfR2 induces iron signaling to hepcidin in the liver and impairs Epo sensitivity in erythroid cells. (B) In an iron-deficient state, TfR2 destabilization prevents iron signaling to hepcidin in the liver and allows enhanced Epo sensitivity in erythroid cells.
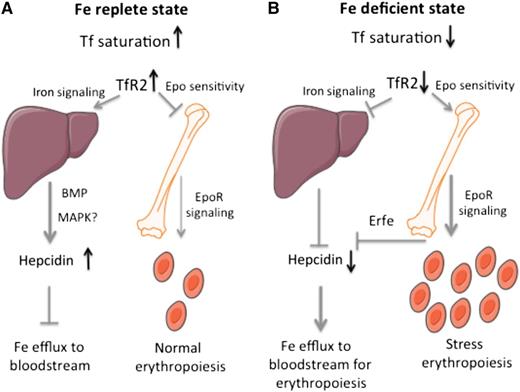
A model for the function of hepatic and erythroid TfR2 in the control of systemic iron metabolism and erythropoiesis. (A) In an iron-replete state, stabilized TfR2 induces iron signaling to hepcidin in the liver and impairs Epo sensitivity in erythroid cells. (B) In an iron-deficient state, TfR2 destabilization prevents iron signaling to hepcidin in the liver and allows enhanced Epo sensitivity in erythroid cells.
Nai et al show that mice lacking bone marrow TfR2 exhibit erythrocytosis and enhanced sensitivity to erythropoietin (Epo). Moreover, their erythroid cell maturation resembles that of iron-deficient wild-type mice. Considering that hepatic TfR2 controls systemic iron traffic, these findings provide a molecular link between iron metabolism and erythropoiesis.
Erythroid cells consume the vast majority (70-80%) of body iron for synthesis of heme, the oxygen-binding cofactor of hemoglobin (Hb). Under physiological conditions, plasma iron is captured by transferrin (Tf), which delivers it to bone marrow erythroblasts and other cell types.2 Diferric transferrin is taken up by TfR1 via an endocytic pathway that is essential for erythropoiesis. TfR2 binds to Tf with a 25-fold lower affinity and cannot substitute for TfR1, even though it is expressed in the erythroid compartment. TfR2 is also expressed in hepatocytes and is mostly known for its regulatory function in systemic iron homeostasis.3 Thus, humans and mice with global or liver-specific inactivation of TfR2 develop hemochromatosis, a disorder of iron overload. Other subtypes of the disease are linked to inactivation of high iron (HFE), an atypical major histocompatibility complex type I protein, or hemojuvelin (HJV), a bone morphogenetic protein (BMP) coreceptor.
Hereditary hemochromatosis is caused by misregulation of hepcidin, the iron regulatory peptide hormone that normally restricts iron fluxes from intestinal enterocytes and tissue macrophages to the bloodstream.4 Humans or mice with a deficiency in TfR2, HFE, or HJV fail to mount appropriate iron-mediated induction of hepcidin. Thus, hepatic TfR2 is widely viewed, together with HFE and HJV, as an upstream regulator of iron signaling to hepcidin. This involves the BMP and possibly additional pathways (such as mitogen-activated protein kinase). TfR2 operates as a sensor of diferric plasma Tf, which protects it against lysosomal degradation, but its exact role on the signaling pathway(s) is not well understood.
Until recently, the function of TfR2 in the erythroid compartment had not received considerable attention, even though genome-wide association studies identified TFR2 polymorphisms that affect hematologic parameters.5,6 A potential role in erythropoiesis was also implied by the identification of TfR2 as a component of the Epo receptor (EpoR) complex in erythroid progenitor cells.7 TfR2 was shown to be crucial for efficient transport of EpoR to the cell surface and for terminal differentiation.7 Another hint was provided by the increased Hb content of hemochromatotic mice with ubiquitous, but not liver-specific, TfR2 deficiency.8 Because both mouse models manifested comparable iron overload, the lack of enhanced hemoglobinization in liver-specific Tfr2−/− mice was likely related to the preservation of erythroid TfR2. Along similar lines, double Tmprss6-Tfr2 knockout mice developed erythrocytosis that was prevented when TfR2 was ablated only in the liver and preserved in erythroid cells.8 These mouse models exhibited a similar degree of iron deficiency due to disruption of the negative hepcidin regulator Tmprss6.
To validate the hypothesis that TfR2 is a crucial erythropoietic regulator, Nai et al developed Tfr2BMKO mice by transplanting bone marrow cells from ubiquitous Tfr2−/− mice to wild-type recipients.1 The chimeric animals lack TfR2 exclusively in erythroid progenitors, the bone marrow cells expressing TfR2. Control chimeras were transplanted with bone marrow from wild-type donors. Tfr2BMKO mice manifested increased red blood cell (RBC) counts and Hb content, as well as reduced mean corpuscular volume and mean corpuscular hemoglobin within 2 to 4 months after bone marrow transplantation.
These responses, which characterize adaptation to iron deficiency, were associated with enhanced terminal erythropoiesis due to reduced apoptosis of late erythroid progenitor cells (basophilic, polychromatic, and orthochromatic erythroblasts), reticulocytes, and RBCs. However, they were not accompanied by increased plasma Epo levels, a hallmark of true iron deficiency. Notably, under conditions of mild dietary iron restriction, erythroid differentiation of control mice was similar to that of iron-replete Tfr2BMKO mice, and as expected, plasma Epo was increased. In iron-poor Tfr2BMKO mice, erythropoiesis was not further modified, and plasma Epo levels remained unchanged, whereas EpoR messenger RNA (mRNA) downregulation during erythroid cell differentiation was delayed. Taken together, these data suggest that the lack of TfR2 confers enhanced Epo sensitivity to erythroid progenitor cells, which is further supported by the induction of Epo target genes (Bcl-xL, Fasl, Serpina3g, Ccng2, Epor, and Erfe). Future studies are expected to clarify whether the lack of erythroid TfR2 directly stimulates EpoR signaling. It is also conceivable that TfR2 may modulate the capacity of EpoR to form productive signaling complexes and/or affect its expression (if EpoR protein levels correspond to mRNA).
Among the Epo downstream targets that are induced in TfR2-deficient erythroid progenitor cells, erythroferrone (Erfe) is of particular importance. This erythroid hormonal regulator suppresses hepcidin expression during stress erythropoiesis, as a homeostatic adaptation to augment iron supply.9 Thus, the induction of Erfe presumably accounts for the reduced hepcidin mRNA levels that are observed in livers of Tfr2BMKO mice. These findings highlight the interconnection between the hepatic and erythroid functions of TfR2 in regulating iron metabolism and erythropoiesis.
A unifying model is depicted in the figure. In an iron-replete state (panel A), TfR2 is stabilized in response to high Tf saturation. Hepatic TfR2 promotes iron signaling to hepcidin to inhibit further iron fluxes to the bloodstream. Erythroid TfR2 restricts Epo sensitivity to limit excessive erythropoiesis. Conversely, in an iron-deficient state (panel B), TfR2 is unstable. Downregulation of hepatic TfR2 inhibits iron signaling to hepcidin to stimulate iron efflux from cells and thereby increase iron supply to erythroblasts. Downregulation of erythroid TfR2 enhances Epo sensitivity to stimulate erythropoiesis; at the same time, it contributes to suppression of hepcidin via Erfe. Thus, the sensor of circulating iron TfR2 couples systemic iron traffic and erythroid utilization. The importance of this network is also emphasized by the existence of an analogous link: the sensor of cellular iron, iron regulatory protein 1, likewise couples systemic iron traffic and erythroid utilization by controlling renal and hepatic Epo production as an upstream regulator of hypoxia inducible factor 2α,10 a major transcriptional inducer of Epo during hypoxaemia or iron deficiency.
Conflict-of-interest disclosure: The author declares no competing financial interests.