In this issue of Blood, Pettirossi et al,1 including Drs Tiacci and Falini, who led the effort in 2011 defining the BRAF-V600E driving mutation in hairy cell leukemia (HCL),2 provide extensive laboratory studies showing that inhibitors of BRAF-V600E and/or mitogen-activated protein kinase kinase (MEK) reach their targets and cause HCL cell death.
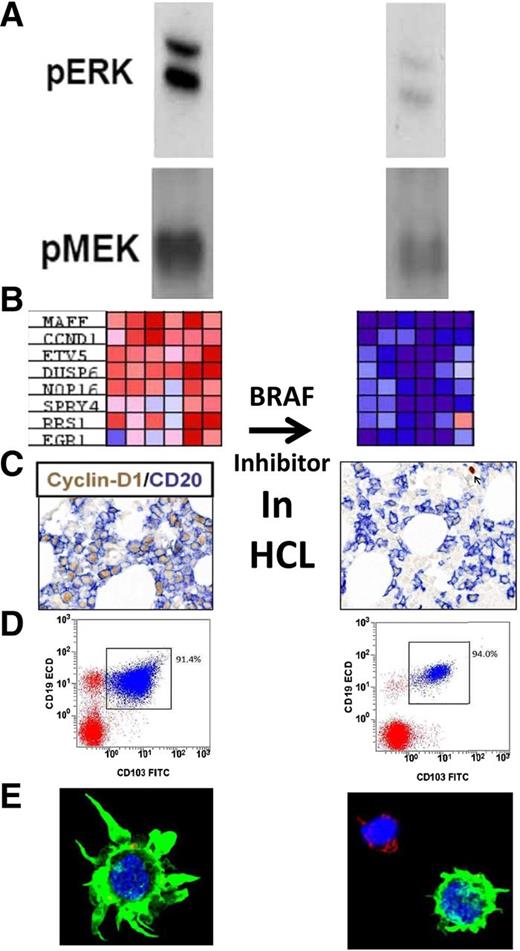
Studies performed by Pettirossi et al to confirm the mechanism of BRAF inhibition in HCL. (Left) Before and (right) after BRAF inhibition with vemurafenib. From top to bottom: (A) phosphorylated ERK and MEK; (B) HCL-related genes with large differences in expression before and after vemurafenib (each column, 1 of 6 patients); (C) bone marrow immunohistochemistry for cyclin D1 and CD20; (D) flow cytometry of the bone marrow aspirate; and (E) confocal microscopy of HCL cells showing loss of hairy morphology. See Figures 1A, 2B, 3A,C, and 4C-D in the article by Pettirossi et al that begins on page 1207.
Studies performed by Pettirossi et al to confirm the mechanism of BRAF inhibition in HCL. (Left) Before and (right) after BRAF inhibition with vemurafenib. From top to bottom: (A) phosphorylated ERK and MEK; (B) HCL-related genes with large differences in expression before and after vemurafenib (each column, 1 of 6 patients); (C) bone marrow immunohistochemistry for cyclin D1 and CD20; (D) flow cytometry of the bone marrow aspirate; and (E) confocal microscopy of HCL cells showing loss of hairy morphology. See Figures 1A, 2B, 3A,C, and 4C-D in the article by Pettirossi et al that begins on page 1207.
These studies, several of which are represented in the figure, include (1) western blots demonstrating that inhibitors prevent phosphorylation of targets MEK and extracellular signal-regulated kinase (ERK) immediately downstream of BRAF, (2) expression profiling after exposure to the BRAF-V600E inhibitor vemurafenib showing strong silencing of genes related to the BRAF-MEK-ERK pathway, (3) downregulation of cyclin D1 and CD25 in HCL cells from patients who received vemurafenib, (4) confocal microscopy showing loss of the hairy morphology as a result of BRAF/MEK inhibition, and (5) dose-dependent cytotoxicity of HCL cells incubated ex vivo with BRAF/MEK inhibitors, including Anxa5 studies, confirming apoptosis as a mechanism of cell death. To investigate why elimination of HCL by this approach is clinically more difficult in bone marrow than in blood, HCL cells during cytotoxicity assays were cocultured with a bone marrow stromal cell line, which blunted the cytotoxicity of BRAF/MEK inhibition.
The BRAF-V600E mutation within the mitogen-activated protein (MAP) kinase pathway is present in many other tumors, most notably malignant melanoma, with nearly 50% of patients expressing BRAF-V600E, but only in HCL are nearly all patients affected.2 An exception is the poor prognosis IGHV4-34 variant, which by immunophenotype can be indistinguishable from classic HCL but lacks BRAF-V600E.3 Several other cases of classic HCL expressing wild-type BRAF have been described and reviewed.4,5 The presence of MEK (MAP2K1) mutations when BRAF is wild type, including both IGHV4-34+ classic HCL and HCL variant (HCLv), exemplifies the importance of the MAP kinase pathway to the pathogenesis of not only HCL but also variants that resemble it.6
Although dabrafenib and trametinib just began being clinically tested for HCL, the effectiveness of vemurafenib in this disease is convincingly documented in case reports and reviews,5,7,8 and several clinical trials are ongoing. As demonstrated again in this study, response often occurs very rapidly, so that by 14 days, significant reductions in HCL cells are already seen in the bone marrow biopsy by immunohistochemistry.1 Studies performed on the HCL cells of 3 patients after treatment with vemurafenib 960 mg orally twice daily showed rapid reductions in cyclin D1 expression. CD25 expression in 1 patient decreased to 62% and 9.7% of baseline by 7 and 14 days, respectively. Improvements in normal blood counts can occur within weeks and complete remission before 2 months after starting vemurafenib.7 Recent experiments showed that the BRAF-V600E mutation in HCL patients is also present in hematopoietic stem/progenitor cells, which develop into the more mature HCL cells, possibly explaining why targeting BRAF-V600E can lead to responses with high durability.9
Although mature clinical data from multiple ongoing studies have not yet been reported, it appears that, although vemurafenib can achieve rapid and clinically valuable remissions, elimination of detectable minimal residual disease (MRD) has not yet been reported even after complete response.7 Currently, the most sensitive standard test for HCL MRD is flow cytometry of the bone marrow aspirate. As shown in this study, the ability of bone marrow stromal cells to block the effect of BRAF inhibition suggests a possible mechanism for persistence of HCL in the bone marrow by flow cytometry after clearance of MRD by other studies. However, persistence of MRD only in the bone marrow is common in HCL after other types of treatments as well.5 Clinical relapse within several months has been documented after partial response to vemurafenib.8 How long patients can be maintained after relapse from complete or partial remission before requiring additional therapy has yet to be reported. Thus far, other treatments for HCL that are associated with elimination of HCL MRD include cladribine alone, at least in a minority of cases, purine analogs combined with rituximab in a majority of cases, and the anti-CD22 recombinant immunotoxin moxetumomab pasudotox.5,10 It will be exciting to determine whether MRD elimination and prevention of relapse in HCL can be achieved by combining targeted approaches and lead to chemotherapy-free initial and salvage treatment of this disease.
Conflict-of-interest disclosure: The author is a coinventor on the National Institutes of Health (NIH) patent for moxetumomab pasudotox, and has a clinical Cooperative Research and Development Agreement through NIH with GlaxoSmithKline connected with dabrafenib and trametinib.