Key Points
Platelet transfusions are frequently administered to hospitalized patients with platelet consumptive/destructive disorders such as TTP, HIT, and ITP.
Platelet transfusions are associated with higher odds of arterial thrombosis and mortality among TTP and HIT patients.
Abstract
While platelets are primary mediators of hemostasis, there is emerging evidence to show that they may also mediate pathologic thrombogenesis. Little data are available on risks and benefits associated with platelet transfusions in thrombotic thrombocytopenic purpura (TTP), heparin-induced thrombocytopenia (HIT) and immune thrombocytopenic purpura (ITP). This study utilized the Nationwide Inpatient Sample to evaluate the current in-hospital platelet transfusion practices and their association with arterial/venous thrombosis, acute myocardial infarction (AMI), stroke, and in-hospital mortality over 5 years (2007-2011). Age and gender-adjusted odds ratios (adjOR) associated with platelet transfusions were calculated. There were 10 624 hospitalizations with TTP; 6332 with HIT and 79 980 with ITP. Platelet transfusions were reported in 10.1% TTP, 7.1% HIT, and 25.8% ITP admissions. Platelet transfusions in TTP were associated with higher odds of arterial thrombosis (adjOR = 5.8, 95%CI = 1.3-26.6), AMI (adjOR = 2.0, 95%CI = 1.2-3.3) and mortality (adjOR = 2.0,95%CI = 1.3-3.0), but not venous thrombosis. Platelet transfusions in HIT were associated with higher odds of arterial thrombosis (adjOR = 3.4, 95%CI = 1.2-9.5) and mortality (adjOR = 5.2, 95%CI = 2.6-10.5) but not venous thrombosis. Except for AMI, all relationships remained significant after adjusting for clinical severity and acuity. No associations were significant for ITP. Platelet transfusions are associated with higher odds of arterial thrombosis and mortality among TTP and HIT patients.
Introduction
The role of platelet transfusions (prophylactic or therapeutic) for managing patients with various platelet consumptive or destructive disorders, including: (1) thrombotic thrombocytopenic purpura (TTP); (2) heparin-induced thrombocytopenia (HIT); and (3) immune thrombocytopenic purpura (ITP) is debated but remains largely undefined and underexplored.
Although platelets are primary mediators of hemostasis at sites of endothelial trauma, there is emerging evidence of their role as mediators in inflammation and pathological thrombogenesis.1-5 It has been hypothesized that platelet transfusions may potentially provoke fatal thrombotic events,6-10 particularly arterial ones, in TTP and HIT.10,11 In TTP, the principal histologic abnormality is proposed to be a “platelet microvascular thrombus.”12,13 In HIT, the underlying physiology is due to the formation of a heparin-dependent anti-platelet factor immune complex resulting in immunologic platelet activation and thrombin generation, and subsequent thrombocytopenia and thrombotic risk.14-16
The role of platelet transfusions in the management of ITP remains unclear. Transfused autologous and allogeneic platelets have shortened survival in ITP, suggesting that platelet transfusions may not be of clinical benefit in this condition.17,18 On the other hand, some case series of platelet transfusions alone, in massive doses, or in conjunction with intravenous immunoglobulin (IVIG), have demonstrated efficacy in increasing platelet counts and preventing or stopping bleeding in ITP patients.19-22 The 2011 American Society of Hematology guidelines recommend that platelet transfusions in ITP should be reserved for catastrophic hemorrhage or concurrent surgeries only.23
Based on the limited available data, the quality of evidence either supporting or negating platelet transfusions in these disorders is generally limited to studies with small sample sizes and resultant inherent methodologic limitations. Thus, evidence-based platelet transfusion guidelines in platelet consumptive disorders are either nonexistent or consensus statements based upon insufficient data. A recent systematic review of the clinical evidence of platelet transfusions by the American Association of Blood Banks platelet transfusion guidelines panel highlighted the paucity of data guiding the use of platelet transfusions in these disorders.24
This study, using a nationally representative hospital discharge database, assessed the current platelet transfusion practices in hospitalized patients with these platelet consumptive disorders and their association, if any, with various clinical outcomes including bleeding, venous and arterial thrombotic events, acute myocardial infarction (AMI), stroke, and overall survival.
Methods
Data source
Data from the Nationwide Inpatient Sample (NIS) for the years 2007-2011 were merged and used for this study. The NIS is the largest all-payer inpatient database in the United States, developed as part of the Healthcare Cost and Utilization Project (HCUP) by the Agency for Healthcare Research and Quality. The NIS uses a multi-staged clustering design to provide a stratified probability sample of 20% of all hospital discharges among US community hospitals (∼1000 to 1100 hospitals per year). Community hospitals, per definition of the American Hospital Association, are all non-federal, short-term general and specialty hospitals, including academic medical centers. Children’s hospitals are also represented in the survey.25 HCUP has selected hospitals in the NIS to be maximally representative and generate nationally representative estimates so as to include 40 to 47 states, and represent 90% to 97% of the US population over the different years.
Information in NIS is de-identified data and includes one primary or principal diagnosis and up to 24 total secondary diagnosis codes, one primary and up to 14 secondary procedure codes, admission and discharge status, demographic information, and hospital characteristics. The principal diagnosis, the main reason that was responsible for the admission, is coded in the first diagnosis field. All listed diagnoses (Dx1 to Dx25) include a principal diagnosis plus additional conditions that either develop during the course of hospitalization or may coexist at the time of admission. Data on laboratory values and pharmacologic therapies administered during the course of an inpatient stay are not available in the NIS. The “unit of analysis” is a hospital discharge and not a specific patient. Thus, the same patient could have had multiple hospitalizations in a specific year and will be captured each time as a separate hospitalization.
Because HCUP–NIS is a de-identified, publicly available data set, informed consent was not obtained. HCUP guidelines in granting the authors access to the NIS data set were followed, and all authors signed the data-use agreement.26
Statistical analyses
Data stratification and analysis was performed using Statistical Analysis Software (SAS), version 9.3.0 (SAS Institute Inc., Cary, NC). In accordance with the HCUP NIS data use agreement, any tabulated data with ≤10 discharges were not reported.26
Using SAS PROC SURVEY and SUDAAN methodology, SAS 9.3.0 callable version, sampling weights were applied to represent all community hospital discharges in the US between 2007 and 2011. As an average between these years, the sampling frame for the NIS is expected to represent 81% of community hospital discharges across 40 to 46 states, and projected to cover 90% to 97% of the US population. Univariate and multivariable logistic regression were performed to assess the independent effect of platelet transfusions on outcomes, including venous and arterial thrombosis and AMI. Variables in multivariable analysis were tested for interaction with a significance threshold level of P < .2. Except for this, all hypothesis testing was two-tailed and P < .05 was considered significant. Multivariable logistic regression was repeated with and without clustering for hospital effects. Clustering the patients by hospitals is expected to be more reliable (95% CI) by taking into account the interfacility correlation of patient outcomes, since outcomes are more likely to be similar within rather than across hospitals27 and has reliably been used in more recent publications using the NIS.28
Identification of discharges
TTP, HIT, and ITP were identified using the International Classification of Diseases, 9th Revision (ICD-9), Clinical Modification diagnosis codes 446.6, 289.84, and 287.31, respectively. For TTP and ITP, we selected the hospital admissions with these conditions as the “primary admitting diagnosis.” TTP and hemolytic uremic syndrome (HUS) are often considered be on the same spectrum of disorders. However, HUS has a separate ICD-9 code, 283.11, and analyses for this study only included TTP hospitalizations. Although HIT can occasionally be a primary diagnosis considered to be mainly responsible for the hospitalization, it is more likely to be a complication that develops during hospitalization (and thus more likely to be one of the secondary diagnoses). Therefore, we a priori elected to include hospitalizations with HIT as one of the top 3 diagnoses for our analysis. Thrombotic events (arterial/venous) were identified using the available listing from the Agency for Healthcare Research and Quality Patient Safety Indicators list (supplemental Appendix 1). Hospitalizations with reported prior “history of thrombosis” identified by diagnosis codes V12.51, V12.52, or V12.55 were excluded. We further excluded hospitalizations that had any thrombosis/thromboembolism listed as the primary admitting diagnosis (ie, being already present at admission). Using the ICD-9 coding, we identified cases of intracranial hemorrhage, gastrointestinal bleed, and/or genitourinary bleed as “bleeding” for the purpose of our analysis (see supplementary material on the Blood Web site).
Sensitivity analyses
We performed the following sensitivity analyses to explore the robustness of our findings: 1) we restricted the hospitalizations with primary diagnoses of “thrombotic microangiopathies” (ICD-9 Clinical Modification code 446.6) only to the admissions with plasma exchange listed as a concurrent procedure during the hospital stay, with a goal to increase the specificity of the TTP diagnosis and treatment; 2) we repeated the analysis for the statistically significant outcomes using admissions with HIT as the primary diagnosis only and as one of the top 2 diagnoses; and 3) we merged the NIS severity files with the core hospital files and adjusted for severity/acuity using All Payer Refined-Diagnosis Related Groups (APR-DRGs) severity and mortality indices. APR-DRG’s are a validated inpatient classification system widely used in the United States, as a case-mix measure and account for severity of illness, risk of mortality, prognosis, treatment difficulty, need for intervention, and resource intensity.29
Results
Between 2007 and 2011, ITP was the most frequently diagnosed platelet consumptive disorder with 79 980 admissions, followed by 10 624 admissions with TTP and 6332 with HIT. Table 1 depicts the national estimates of the number of hospitalizations per year for each disease entity. Of note, no estimates were available for HIT before 2008 since an independent ICD-9 code for HIT was not identified until 2007 (thus, there is an appreciable difference between the number of reported hospitalizations with HIT between 2008 and 2009). Overall, hospitalizations per year were fairly steady across the 5 years between 2007 and 2011. The majority of hospitalized patients were females (66.6%) for TTP, and males for ITP (56.6%) and HIT (51.3%). Only a small minority of admissions were children (aged <18 years) for all disease types (1.9% TTP, 0.5% HIT, and 20.9% ITP). The most common race was white for each disease entity (Table 1).
Platelet transfusions were reported in 10.1% of all hospitalizations for TTP, 7.1% for HIT, and 25.8% for ITP. In each condition, platelets were more commonly administered to adults than children (P < .01). The most common procedure performed in admissions with TTP was plasma exchange (64% of admissions). A simple plasma infusion was reported in 32.3% of TTP admissions. In 75.3% of TTP admissions, either plasma exchange or plasma infusion was performed. In HIT and ITP, the most common procedures were red blood cell (RBC) transfusion (16.6%) and IVIG administration (20.9%), respectively (Table 1).
Table 2 lists the major complications/comorbidities documented during hospitalization for each disease type. TTP, HIT, and ITP had a bleeding event reported in 13.7%, 5.7%, and 11.5% of the admissions, respectively. In TTP and ITP, the most common bleeding type was genitourinary bleeding (8.6% and 6.1%, respectively), whereas in HIT the most frequent bleeding type was gastrointestinal bleeding (2.7%). Central nervous system (CNS) bleeding was reported at ∼1% in TTP, ITP, and HIT.
Among thrombotic complications/comorbidities, HIT had the highest percentage of thrombotic events with 20.6% (17.6% venous, 3.4% arterial, 0.4% of admissions reported both arterial and venous thromboses), TTP with 4.1% (3.8% venous and 0.3% arterial), and ITP with 0.9% (0.8% venous and 0.1% arterial). Likewise, HIT also had the highest frequency of AMI events (7.1%), followed by TTP (5.1%) and ITP (0.5%). TTP admissions had the highest percentage of stroke (5.2%), followed by HIT (2.3%) and ITP (0.3%) (Table 2).
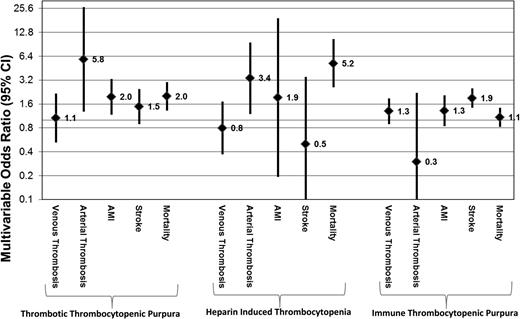
There were significantly higher odds of platelet transfusion in patients with reported bleeding (either CNS, gastrointestinal bleeding, or genitourinary bleeding) in each of the disease entities after age and gender adjustment: TTP (adjOR = 2.3, 95% CI = 1.6-3.2), HIT (adjOR = 5.5, 95% CI = 2.3-12.9), and ITP (adjOR = 5.1, 95% CI = 4.2-6.1) (Table 3). Platelet transfusions in TTP patients were associated with increased odds of arterial thrombosis even after adjusting for age and gender (adjOR = 5.8, 95% CI = 1.3-26.6), and higher odds for AMI (adjOR = 2.0, 95% CI = 1.2-3.3) but not increased odds of venous thrombosis (adjOR = 1.1, 95% CI = 0.5-2.2). Platelet transfusions in patients admitted with HIT were also associated with a higher age and gender adjusted odds of arterial (adjOR = 3.4, 95% CI = 1.2-9.5) but not venous thrombosis (adjOR = 0.8, 95% CI = 0.4-1.7). Platelet transfusions among ITP patients were not significantly associated with venous thrombosis, arterial thrombosis, AMI, or stroke after adjustment for age and gender (Table 3; Figure 1).
Multivariable OR and 95% CI of platelet transfusions and various comorbidities/in-hospital mortality in hospitalized patients with TTP, HIT, and ITP. Data are representative of a weighted nationally representative sample of US community hospitals across 5 years (NIS, 2007 to 2011).
Multivariable OR and 95% CI of platelet transfusions and various comorbidities/in-hospital mortality in hospitalized patients with TTP, HIT, and ITP. Data are representative of a weighted nationally representative sample of US community hospitals across 5 years (NIS, 2007 to 2011).
All-cause in-hospital mortality for TTP, HIT, and ITP admissions was 8.8%, 3.4%, and 1.4%, respectively (Table 4). In admissions for TTP and HIT, platelet transfusions were associated with significantly higher odds of all-cause mortality after adjusting for age and gender with an adjOR of 2.0 (95% CI = 1.3-3.0) and 5.2 (95% CI = 2.6-10.5) for TTP and HIT, respectively. Platelet transfusions did not have any significant association with mortality in ITP admissions after adjusting for age and gender with an adjOR of 1.1 (95% CI = 0.9-1.4) (Table 4).
Number needed to harm
With HIT, there are 6.9% arterial thromboses in those with platelet transfusions vs 3.1% in those without (ie, 3.8% extra thrombosis with platelet transfusions). Thus, for every 26 patients receiving platelet transfusions, we expect 1 additional arterial thrombosis. In TTP, there are 1.8% arterial thromboses in those with platelet transfusions vs 0.3% in those without. Thus, for every 75 patients receiving platelet transfusions, we expect 1 additional arterial thrombosis.
Sensitivity analyses
Restricting the thrombotic microangiopathies to admissions with concurrent plasma exchange as a marker for increased specificity of TTP diagnosis and treatment, we found similar results: platelet transfusions were associated with higher age and gender adjusted odds of arterial thrombosis (adjOR = 6.4, 95% CI = 1.1-39.3), as well as mortality (adjOR = 2.2, 95% CI = 1.3-3.9). We also performed additional analyses using HIT as: (1) the primary admitting diagnosis (n = 1445) and (2) one of the top 2 discharge diagnoses (n = 3031). Platelet transfusions were associated with higher age and gender adjusted odds of arterial thrombosis in admissions with a primary admission diagnosis of HIT (adjOR = 4.1, 95% CI = 0.9-19.3) and admissions using HIT as one of the top 2 diagnoses (adjOR = 4.1, 95% CI = 1.0-16.8) (supplemental Table 1).
After adjusting for severity/acuity using APR-DRG severity indices, the relationship for platelet transfusions in TTP with arterial thrombosis (adjOR = 10.8, 95% CI = 1.1-82.8), as well as mortality (adjOR = 2.5, 95% CI = 1.2-5.33) remained statistically significant and with a similar effect size. However, the risk of AMI with platelet transfusion did not remain significant after this adjustment (adjOR = 2.1, 95% CI = 0.8-5.3). Likewise, for HIT admissions, after adjusting for severity/acuity risk, there remained a significant association between platelet transfusions and arterial thrombosis (adjOR = 2.8, 95% CI = 1.01-7.9) and mortality (adjOR = 3.8, 95% CI = 1.7-8.2), respectively.
Discussion
In the absence of established efficacy of platelet transfusions in various platelet consumptive disorders, as well as the inability to rule out harm, the justification of their use is highly controversial. Due to lack of high quality data, there are no definite evidence-based practice guidelines for platelet transfusions in these conditions. Additionally, there is no literature supporting a specific platelet count to serve as a transfusion threshold metric. Consequently, there remains a large heterogeneity in the use of platelet transfusions in these disorders with the decision to transfuse based on individual provider preference or institutional recommendations, at best.
Our study provides the first comprehensive national estimate of platelet transfusion practices with the platelet consumptive disorders TTP, HIT, and ITP. We hereby present results from the largest available inpatient database supporting a significant association between platelet transfusions and arterial thrombotic events in TTP and HIT, independent of age, gender, and clinical severity/acuity. Notably, no relationship was seen between platelet transfusions and “venous” thrombotic events in either TTP or HIT. Additionally, platelet use was associated with both documented bleeding and higher mortality, likely implying their utilization in sicker hospitalized patients. Platelet transfusions in TTP patients were also associated with moderately high odds of AMI. However, among ITP patients, platelet transfusions were not associated with venous or arterial thrombosis, AMI, or any improvement in survival after adjustment for age and gender. It is important to note that these reported events occurred in patients who received platelets during their hospitalization; however, we do not know the temporality of events and if the transfusion preceded the event.
Platelet transfusions are typically not recommended for patients with TTP because they have been hypothesized to provoke fatal thrombotic events.6-8 These published reports have held credibility due to: (1) biologic basis of the hypothesis; (2) identification of a close temporal relationship between platelet transfusions and adverse outcomes; and (3) autopsy analysis revealing that platelets were the principal component of the thrombotic lesion in TTP patients.6-8,12 The generally accepted practice has been to restrict platelet transfusions only to life-threatening bleeds, and in fact, some clinical recommendations institute low-dose anti-platelet agents concurrent with platelet count recovery.30-32 A systematic review and analysis of data from the Oklahoma TTP-HUS Registry could neither rule in nor rule out adverse outcomes from platelet transfusions and concluded that there was “uncertain evidence of harm.”33 Our large data set suggests that platelets may be associated with 6 times higher odds of developing arterial (but not venous) thrombosis and 2 times higher odds of AMI, in a population with no reported prior history of thrombosis after adjusting for age and gender. Association with arterial but not venous thrombosis and AMI both add credence to the proposition of “platelet vascular thrombi” as the causal pathology in TTP’s clinical manifestations. This study is the first to provide evidence supporting this association beyond the aforementioned evidence based on case reports.
In HIT, the formation of a heparin-dependent anti-platelet factor antibody results in immune-mediated activation of platelets and increased thrombotic risk, particularly arterial, due to increased thrombin generation.9,10 The benefit of platelets is questionable as transfusions have not been associated with sustained platelet recovery.10,34 In a large retrospective review of over 400 patients, Greinacher et al proposed the magnitude of decline in platelet count as one of the major risk factors for thrombo-embolic complications.35 Other studies have also proposed thrombosis risk in HIT to be associated with the degree of thrombocytopenia.16,35,36 This is very important as the lower platelet counts may trigger platelet transfusions, and in turn, predispose to thrombosis. The 2012 American College of Chest Physicians clinical practice guidelines for HIT do not recommend correcting the platelet count for any specific threshold, and recommend restricting platelet transfusions to clinical bleeding or prophylaxis only for high-risk invasive procedures citing that “while there is no direct evidence supporting use of platelets in these disorders, the evidence is also too limited to support the safety of platelet transfusions” (Grade 2C, which is a very weak recommendation).34 While small case series negate the association,37,38 there is recent contrary evidence questioning the efficacy of platelet transfusions in producing sustained platelet increments or controlling bleeding, as well as showing a positive temporal relationship with catheter-associated thrombosis.39 In sum, expert opinion about platelet transfusions continue to support the avoidance of prophylactic transfusions in HIT patients, with consideration given to individual cases with a high risk of bleeding or significant clinical bleeding.40 This study provides the first nationwide estimates of platelet transfusion practices in HIT and shows an association with platelet transfusions and arterial but not venous thrombi.
For ITP, the effectiveness of platelet transfusions has never been established.17,18 Most published guidelines recommend that platelet transfusions in ITP be limited to clinical situations complicated by life-threatening hemorrhage or concurrent surgical procedures.23,41-45 The occasional case reports of thrombotic events with ITP have been concurrent with administration of thrombopoietic agonists and not with platelet transfusions.46 Our current study also reports no adverse thrombotic events, AMI, or stroke with platelet administration in ITP admissions. Also, platelets were not associated with higher odds of mortality in ITP patients after adjustment for age and gender.
This study uses 5 years of nationally representative data from the largest available all-payer hospitalization database. It is however, subject to a few limitations inherent in the secondary analysis of any administrative data set for evaluation of clinical and health care utilization outcomes. The ICD-9 coding is carried out primarily for billing purposes and it is not per se possible to verify its accuracy. Reassuringly, the ICD-9 hospital discharge codes have been demonstrated previously in studies exploring other outcomes to be adequately sensitive.47 Prior medical record audit studies have specifically shown HCUP–NIS coding to have both adequate sensitivity and specificity.48 NIS has also previously been extensively validated against the National Hospital Discharge Survey and the Medicare Provider Analysis and Review file.49 Also, there is increasingly successful utilization of registry data and databases using validated claims-based diagnoses to study outcomes for relatively uncommon disorders such as TTP and ITP, and serve as excellent prototypes for research and patient care.50,51
Importantly, the temporality of the various clinical outcomes studied (except mortality) in relation to platelet transfusions cannot be assessed from this data set. Although we see increased association of platelet transfusions with bleeding complications in the data, we cannot exactly determine the benefit of platelet transfusion in improving/preventing bleeding outcomes. Additionally, some of the diagnoses may lack specificity (eg, not all TTP patients had plasma exchange therapy or plasma exchange may have been used only transiently in some patients). Also, since this report includes data from large academic centers as well as non-academic centers, there is a possibility that the true magnitude of the association between thrombotic complications and platelet transfusions among patients with severe ADAMTS13 deficiency might be under reported. However, all the sensitivity analyses performed confirmed the primary results. Another limitation is the lack of laboratory data in NIS, particularly the platelet count, which might be an important determinant of platelet transfusions trigger. Specifically, as outlined above, there could be confounding from the degree of thrombocytopenia but this was not addressed in this study.
In conclusion, platelet transfusions are frequently administered to patients with platelet consumptive disorders, including TTP, HIT, and ITP. These data suggest that platelet transfusions are common and likely administered to sicker patients and those with clinical bleeding, and may be associated with higher odds of arterial thrombosis (but not venous thrombosis) and mortality among hospitalized patients with a diagnosis of TTP and HIT. Whether the platelet transfusions were directly responsible for these events or if they served as a surrogate marker for severity of illness cannot be absolutely determined. However, the results remained significant after adjusting for clinical severity/acuity. Future mechanistic studies exploring these relationships are warranted for these disorders. However, more reliable evidence from a randomized controlled trial may be very hard to obtain in these rare disorders with limited use of platelet transfusions. Until mechanistic studies or trials are available to demonstrate the efficacy and risks of platelet transfusions in TTP and HIT, platelets should be considered to be relatively contraindicated and used only for emergencies, invasive procedures, surgeries, or severe or life-threatening bleeding refractory to other therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by a grant from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (1K23AI093152-01A1) (A.A.R.T.).
Authorship
Contribution: R.G., A.A.R.T., P.M.N., C.M.T., L.K., and K.E.K. were involved in the conception and design of the manuscript; R.G. and A.A.R.T. analyzed and interpreted the data; R.G. collected and assembled the data; and all authors were involved in manuscript writing and final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aaron A. R. Tobian, Department of Pathology, Johns Hopkins University, Carnegie 437, 600 N. Wolfe St., Baltimore, MD 21287; e-mail: atobian1@jhmi.edu.