Key Points
Iron deficiency results in symptom improvement in CEP and could be considered a novel therapeutic approach for this disease.
CEP marrow cells demonstrated improved growth and erythroid differentiation in vitro under conditions of relative iron restriction.
Abstract
Congenital erythropoietic porphyria (CEP) is an autosomal recessive disorder of heme synthesis characterized by reduced activity of uroporphyrinogen III synthase and the accumulation of nonphysiologic isomer I porphyrin metabolites, resulting in ineffective erythropoiesis and devastating skin photosensitivity. Management of the disease primarily consists of supportive measures. Increased activity of 5-aminolevulinate synthase 2 (ALAS2) has been shown to adversely modify the disease phenotype. Herein, we present a patient with CEP who demonstrated a remarkable improvement in disease manifestations in the setting of iron deficiency. Hypothesizing that iron restriction improved her symptoms by decreasing ALAS2 activity and subsequent porphyrin production, we treated the patient with off-label use of deferasirox to maintain iron deficiency, with successful results. We confirmed the physiology of her response with marrow culture studies.
Introduction
Congenital erythropoietic porphyria (CEP) is an autosomal recessive disorder resulting from mutations in the uroporphyrinogen III synthase (UROS) gene, which encodes the fourth enzyme in the heme synthetic pathway.1 Reduced UROS activity leads to nonenzymatic conversion of hydroxymethylbilane to isomer I porphyrin metabolites, which accumulate in late erythroid precursors, reticulocytes, and red cells, resulting in ineffective erythropoiesis, hemolysis, and splenomegaly and which disseminate into tissues such as skin to cause severe photosensitivity. Management is largely comprised of sun avoidance, splenectomy, and supportive measures, and in some patients, hematopoietic stem cell transplantation has been effective.2-4 More than 45 mutations in UROS have been identified,5 and disease severity correlates with the degree that enzymatic activity is reduced.6-9 As evidence that additional factors may influence the disease phenotype, a gain-of-function mutation in 5-aminolevulinate synthase 2 (ALAS2), which encodes the first and rate-limiting enzyme in the heme biosynthetic pathway, has been associated with more severe CEP symptoms.10 This raises the question of whether decreased ALAS2 activity would lessen disease severity by decreasing porphyrin production. Deficiency of iron impedes heme synthesis upstream of UROS by decreasing ALAS2 translation via binding of an iron regulatory protein to an iron-responsive element in the 5′ untranslated region of ALAS2 mRNA, suggesting a possible therapeutic role for iron restriction in the treatment of erythropoietic porphyrias.11,12
Study design
Bone marrow cell cultures
Marrow was obtained postmortem from CEP patient 1 with parental consent. Marrow was also obtained from her younger sister (patient 2) with institutional review board approval. Mononuclear cells were cultured adapting the protocol of Giarratana et al13 by decreasing erythropoietin from 3 to 2 IU/mL and extending step 1 from 7 to 10 days. Cultures contained 5% plasma and varying ratios of holo-transferrin (holo-Tf) and apo-transferrin (apo-Tf) (Sigma), yielding a range of available iron from 0.54 to 1.52 μM with 100% apo-Tf up to 8.54 to 9.52 μM with 100% holo-Tf.
Flow cytometry
Cells were analyzed and sorted by BD FACS Canto II or FACAria flow cytometers with Cell Quest software on culture days 10, 13, and 17. Anti-CD3, anti-C11b, and anti-CD19 were used to deplete nonerythroid cells, and anti-CD36, anti-CD235 (glycophorin A), and anti-CD71 (all from BD Pharmingen) were used to monitor erythroid differentiation. An Annexin V-FITC Apoptosis Detection Kit I with propidium iodide staining solution was used for apoptosis assays.
RNA and protein studies
Total RNA was isolated using TRIzol (Ambion), and cDNA was synthesized with reverse transcriptase in SuperScript First-Strand Synthesis System (Bio-Rad). Multiplex quantitative polymerase chain reaction was performed with KAPA Probe Fast Bio-Rad iCycler qPCR kits. Human cDNA clones (OriGene) were used as standards. Probes were labeled with fluorescein amidite, hexachlorofluorescein, and cyanine 5 (Integrated DNA Technologies). The results were expressed as copy numbers normalized by β-actin in 50 ng total RNA. Western blots of cell lysates were probed with rabbit anti-ALAS2 (Santa Cruz), mouse monoclonal anti-β-globin (Abcam), and anti-β-actin (Sigma) antibodies.
Results and discussion
Clinical data
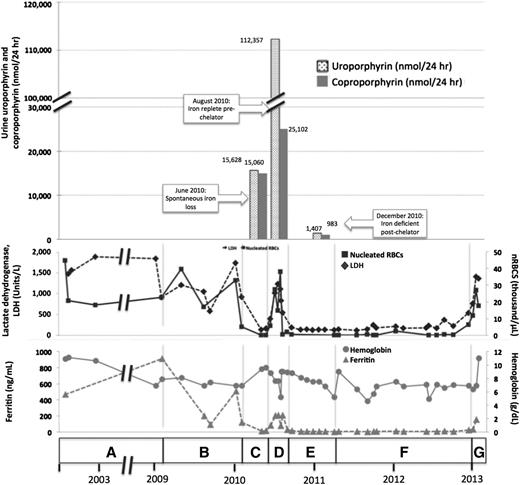
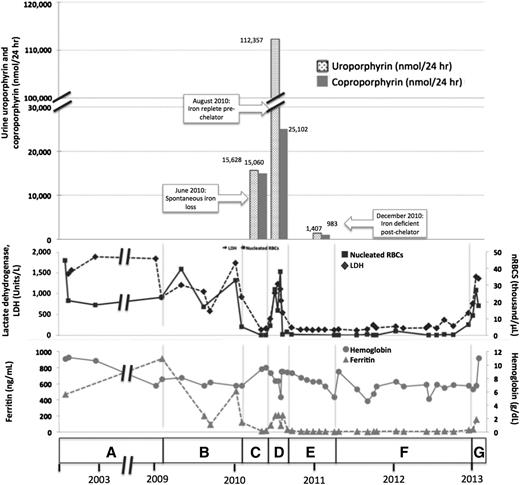
A female of Alaskan Native descent (patient 1) was diagnosed with CEP at 12 months of age after presenting with red urine, discolored teeth, and blisters. A younger sister (patient 2) would later be diagnosed with CEP. Genetic testing revealed compound heterozygosity for previously described C73R and A104V mutations in UROS. Since childhood, disease complications included chronic hemolysis, with continued lactate dehydrogenase (LDH) >1000 U/L and nucleated red blood cells at ∼33.0 × 103 cells/μL, and severe photosensitivity with scarring. These were managed with sun avoidance and supportive measures, including blood transfusions and then splenectomy. At age 32, in the setting of iron deficiency from gastrointestinal bleeding, we noted a spontaneous improvement in her photosensitivity and hemolysis (LDH decreased to 138 U/L and nucleated red blood cells to 2.0 × 105 cells/μL). Symptoms worsened again on resolution of the bleeding. Hypothesizing that iron restriction had improved symptoms by decreasing ALAS2 activity, we treated her with deferasirox. With chelation, total urine porphyrins decreased from 108 364 to 5896 μg/24 hours, markers of hemolysis normalized, and photosensitivity again improved. Her full clinical course and laboratory findings are shown in Figure 1 and supplemental Tables 1 and 2 available on the Blood Web site.
Correlation of clinical and laboratory findings in patient 1. (A) A female of Alaskan Native descent (patient 1) was diagnosed with CEP at 12 months of age after presenting with red urine, discolored teeth, and blisters. A younger sister (patient 2) would also later be diagnosed with CEP. Genetic testing revealed compound heterozygosity for previously described C73R and A104V mutations in UROS. Since childhood, disease complications included chronic hemolysis and severe photosensitivity with scarring, which were managed with sun avoidance and supportive measures including blood transfusions. Patient 1 underwent laparoscopic splenectomy in February 2002 at the age of 25 years. Baseline laboratory parameters include an LDH that ranged from 906 to 1885 U/L (reference range, 80-190 U/L), nucleated red blood cells (nRBCs) between 17.1 and 44.9 × 103 cells/μL (reference, 0.0 × 103 cells/μL), and a reticulocyte count of 195 billion cells/L (reference, 20-65 billion cells/L), reflecting chronic hemolysis. The hemoglobin level ranged from 6.9 to 10.9 g/dL (reference range, 11.5-15.5 g/dL), and the patient’s ferritin level was 470 ng/mL in 2002 (reference range, 10-180 ng/mL). Laboratory values are provided in detail in supplemental Table 1. (B) In February 2009, the ferritin level increased to 910 ng/mL as a result of blood transfusions. Deferasirox was administered to treat iron overload and was discontinued after ferritin level decreased to 93 ng/mL in September 2009. The ferritin level increased again to 508 ng/mL by January 2010. (C) In May 2010, a spontaneous reduction in serum ferritin level to 13 ng/mL was noted and was attributed to occult gastrointestinal (GI) bleeding. Concurrently, the patient reported a dramatic improvement in photosensitivity and a normalization in urine color. Markers of hemolysis also improved, as shown, with a reduction in LDH level to 138 U/L and a marked reduction in circulating nRBCs to 0.2 × 103 cells/μL. Hemoglobin level increased from 6.9 to 9.4. Reticulocyte count decreased to 55 billion cells/L (data not shown). Quantitative analysis of urine porphyrins demonstrated uroporphyrin at 15 628 nmol/24 hours and coproporphyrin at 15 060 nmol/24 hours. Prior values are not available for comparison, and full data regarding the porphyrin fractions are available in supplemental Table 2. (D) Resolution of GI bleeding was accompanied by a rise in serum ferritin level to 208 ng/mL and a concordant worsening of photosensitivity and darkening of urine color. Similarly, LDH level increased to 540 U/L, reticulocyte count increased markedly to 163 billion cells/L, and nRBCs increased to 4.3 × 103 cells/μL. Repeat quantitative analysis of urine porphyrins at this time demonstrated a significant increase in both uroporphyrin (112 357 nmol/24 hours) and coproporphyrin (25 102 nmol/24 hours). (E) In August 2010, based on the correlation between iron status, laboratory parameters, and photosensitivity, we initiated a trial of deferasirox to induce and maintain an iron-deficient state. Deferasirox was initially dosed at 500 mg daily and then in November 2010 was adjusted to 500 mg 3 times weekly to target a ferritin level range of 10 to 15 ng/mL. With deferasirox, the serum ferritin level dropped to 16 ng/mL over 2 months, and the patient again reported an improvement in her quality of life with reduced photosensitivity. As further evidence of the efficacy of deferasirox, an impressive reduction in uroporphyrin to 1470 nmol/24 hours and coproporphyrin to 983 nmol/24 hours was seen on repeat quantitative analysis of urine porphyrins 4 months after chelation began. In addition, total (unfractionated) urine porphyrins decreased from 108 364 μg/24 hours in June 2010 to 5896.3 μg/24 hours in December 2010. Concurrently, LDH normalized to a level of 135 U/L, reticulocyte count decreased to 47 billion/L, nRBCs remained between 0.14 and 0.68 × 103 cells/μL, and hemoglobin level remained between 6.8 and 9.0 g/dL without transfusional support. (F) In April 2011, patient 1 developed recurrent upper GI bleeding, deferasirox was discontinued, and red blood cell transfusions were administered. Over the ensuing 2 years, numerous repeat endoscopies showed persistent gastric erosions and ulcerations despite ongoing use of a proton pump inhibitor. As a result of the ongoing iron losses, ferritin level remained <15 ng/mL, and there was continued control of CEP symptoms and stabilization of laboratory markers of hemolysis. (G) In January 2013, the patient’s serum ferritin increased abruptly, consistent with resolution of GI bleeding. Her photosensitivity suddenly worsened, accompanied by a rise in LDH level to 1403 U/L, nRBCs to 6.4 × 103 cells/μL, and reticulocytes to 118 billion cells/L. Although not known to be a complication of CEP, the patient developed liver disease. Workup, including liver biopsy, failed to discern its etiology. This contributed to a delay in restarting deferasirox when her iron levels began to increase again. In early 2013, at age 35, the patient died of complications of liver failure, hepatorenal syndrome, and hemolysis after enjoying nearly 3 years of reduced symptom severity and a considerable improvement in her quality of life. Microscopic examination of the liver at the time of autopsy demonstrated areas of marked sinusoidal congestion and dilatation containing aggregates of erythroid precursors consistent with diffuse intrasinusoidal extramedullary hematopoiesis. Extensive patchy fibrosis was present, without regenerative nodules or cirrhosis. Importantly, there was no evidence of polarizable material to suggest porphyrin metabolite accumulation. Sadly, the patient’s sister (patient 2) also died, in 2011, from sudden cardiac death in the setting of pulmonary hypertension. A similar trial of chelation was not initiated in her case because she was unable to obtain insurance coverage for the off-label use of deferasirox. Patient 2’s clinical course is further described in supplemental Appendix 1.
Correlation of clinical and laboratory findings in patient 1. (A) A female of Alaskan Native descent (patient 1) was diagnosed with CEP at 12 months of age after presenting with red urine, discolored teeth, and blisters. A younger sister (patient 2) would also later be diagnosed with CEP. Genetic testing revealed compound heterozygosity for previously described C73R and A104V mutations in UROS. Since childhood, disease complications included chronic hemolysis and severe photosensitivity with scarring, which were managed with sun avoidance and supportive measures including blood transfusions. Patient 1 underwent laparoscopic splenectomy in February 2002 at the age of 25 years. Baseline laboratory parameters include an LDH that ranged from 906 to 1885 U/L (reference range, 80-190 U/L), nucleated red blood cells (nRBCs) between 17.1 and 44.9 × 103 cells/μL (reference, 0.0 × 103 cells/μL), and a reticulocyte count of 195 billion cells/L (reference, 20-65 billion cells/L), reflecting chronic hemolysis. The hemoglobin level ranged from 6.9 to 10.9 g/dL (reference range, 11.5-15.5 g/dL), and the patient’s ferritin level was 470 ng/mL in 2002 (reference range, 10-180 ng/mL). Laboratory values are provided in detail in supplemental Table 1. (B) In February 2009, the ferritin level increased to 910 ng/mL as a result of blood transfusions. Deferasirox was administered to treat iron overload and was discontinued after ferritin level decreased to 93 ng/mL in September 2009. The ferritin level increased again to 508 ng/mL by January 2010. (C) In May 2010, a spontaneous reduction in serum ferritin level to 13 ng/mL was noted and was attributed to occult gastrointestinal (GI) bleeding. Concurrently, the patient reported a dramatic improvement in photosensitivity and a normalization in urine color. Markers of hemolysis also improved, as shown, with a reduction in LDH level to 138 U/L and a marked reduction in circulating nRBCs to 0.2 × 103 cells/μL. Hemoglobin level increased from 6.9 to 9.4. Reticulocyte count decreased to 55 billion cells/L (data not shown). Quantitative analysis of urine porphyrins demonstrated uroporphyrin at 15 628 nmol/24 hours and coproporphyrin at 15 060 nmol/24 hours. Prior values are not available for comparison, and full data regarding the porphyrin fractions are available in supplemental Table 2. (D) Resolution of GI bleeding was accompanied by a rise in serum ferritin level to 208 ng/mL and a concordant worsening of photosensitivity and darkening of urine color. Similarly, LDH level increased to 540 U/L, reticulocyte count increased markedly to 163 billion cells/L, and nRBCs increased to 4.3 × 103 cells/μL. Repeat quantitative analysis of urine porphyrins at this time demonstrated a significant increase in both uroporphyrin (112 357 nmol/24 hours) and coproporphyrin (25 102 nmol/24 hours). (E) In August 2010, based on the correlation between iron status, laboratory parameters, and photosensitivity, we initiated a trial of deferasirox to induce and maintain an iron-deficient state. Deferasirox was initially dosed at 500 mg daily and then in November 2010 was adjusted to 500 mg 3 times weekly to target a ferritin level range of 10 to 15 ng/mL. With deferasirox, the serum ferritin level dropped to 16 ng/mL over 2 months, and the patient again reported an improvement in her quality of life with reduced photosensitivity. As further evidence of the efficacy of deferasirox, an impressive reduction in uroporphyrin to 1470 nmol/24 hours and coproporphyrin to 983 nmol/24 hours was seen on repeat quantitative analysis of urine porphyrins 4 months after chelation began. In addition, total (unfractionated) urine porphyrins decreased from 108 364 μg/24 hours in June 2010 to 5896.3 μg/24 hours in December 2010. Concurrently, LDH normalized to a level of 135 U/L, reticulocyte count decreased to 47 billion/L, nRBCs remained between 0.14 and 0.68 × 103 cells/μL, and hemoglobin level remained between 6.8 and 9.0 g/dL without transfusional support. (F) In April 2011, patient 1 developed recurrent upper GI bleeding, deferasirox was discontinued, and red blood cell transfusions were administered. Over the ensuing 2 years, numerous repeat endoscopies showed persistent gastric erosions and ulcerations despite ongoing use of a proton pump inhibitor. As a result of the ongoing iron losses, ferritin level remained <15 ng/mL, and there was continued control of CEP symptoms and stabilization of laboratory markers of hemolysis. (G) In January 2013, the patient’s serum ferritin increased abruptly, consistent with resolution of GI bleeding. Her photosensitivity suddenly worsened, accompanied by a rise in LDH level to 1403 U/L, nRBCs to 6.4 × 103 cells/μL, and reticulocytes to 118 billion cells/L. Although not known to be a complication of CEP, the patient developed liver disease. Workup, including liver biopsy, failed to discern its etiology. This contributed to a delay in restarting deferasirox when her iron levels began to increase again. In early 2013, at age 35, the patient died of complications of liver failure, hepatorenal syndrome, and hemolysis after enjoying nearly 3 years of reduced symptom severity and a considerable improvement in her quality of life. Microscopic examination of the liver at the time of autopsy demonstrated areas of marked sinusoidal congestion and dilatation containing aggregates of erythroid precursors consistent with diffuse intrasinusoidal extramedullary hematopoiesis. Extensive patchy fibrosis was present, without regenerative nodules or cirrhosis. Importantly, there was no evidence of polarizable material to suggest porphyrin metabolite accumulation. Sadly, the patient’s sister (patient 2) also died, in 2011, from sudden cardiac death in the setting of pulmonary hypertension. A similar trial of chelation was not initiated in her case because she was unable to obtain insurance coverage for the off-label use of deferasirox. Patient 2’s clinical course is further described in supplemental Appendix 1.
Pathophysiologic studies
Marrow mononuclear cells from the CEP patients and a normal donor were cultured under conditions optimizing erythroid differentiation (Figure 2A-B; supplemental Figure 1A-B). Both CEP and normal erythroid cells matured fully, as determined by their sequential expression of CD36 and glycophorin A (GlyA). However, at culture day 10, there were fewer percentages and absolute numbers of CEP cells in stages III (CD36+,GlyA+) and IV (CD36−,GlyA+) than normal cells, suggesting that CEP cells died in stage III, when heme synthesis intensifies and concentrations of isomer I porphyrin metabolites would be expected to increase in the CEP cells. Because the percentage of apoptotic cells at day 7 (when most erythroid cells transitioned from stages II to III) was increased (8.9% in patient 2 cultures vs 5.4% in control cultures), a component of cell death is attributable to apoptosis. In addition, some cell death may result from exposing erythroid precursors with excess porphyrins to ambient light during the analytic steps.
Erythroid cell growth and maturation of CEP and normal bone marrow. (A) Flow cytometric profiles of control and CEP marrow cells cultured in standard iron-replete conditions (100% holo-Tf [holo-transferrin]; 8.54-9.52 μM iron). Although the erythroid cells from CEP patient 2 fully matured from stages I to IV (defined by the sequential gain of CD36 and then GlyA expression and the subsequent loss of CD36 expression), there were lower percentages of erythroid cells in stage III on day 10 (55.4% vs 64.4% in control cultures) and in stage IV on days 13 and 17 (33.1% and 73.0% vs 57.8% and 83.6%, respectively). Similar results were also seen in the studies of marrow cells from CEP patient 1 (supplemental Figure 1A). (B) Absolute numbers of erythroid cells in cultures containing 100% holo-Tf. Cultures of marrow cells from CEP patient 2 at day 10 have fewer cells than control cultures. This is mainly due to the decreased numbers of erythroid cells in stage III (19.0 × 105 vs 29.6 × 105), suggesting that CEP erythroid cells die when transiting to or in stage III. All cultures were initiated (day 0) with 2.0 × 106 bone marrow mononuclear cells. Similar results were also seen in marrow culture studies of CEP patient 1 (supplemental Figure 1B). (C) Total number of CEP erythroid cells on culture day 10 in media containing different ratios of holo-Tf to apo-Tf, as stated in Study design. The number of erythroid cells in cultures containing 100% holo-Tf was arbitrarily designated as 10 so that data from CEP patients 1 and 2 could be combined and both increases and decreases reported. Maximal erythroid growth occurred with 50% holo-Tf /50% apo-Tf. In this condition of partial iron restriction, there was a 2.5 ± 0.4-fold increase in the numbers of erythroid cells vs 0.9 ± 0.5- to 1.2 ± 0.1-fold changes in the other conditions. In contrast, the numbers of normal erythroid cells on culture day 10 did not change significantly when iron delivery is restricted. (D) Iron restriction also improves the erythroid maturation of CEP marrow cells. On culture day 10, a greater proportion of CEP patient 2’s cells were GlyA+ (stages III and IV) in the cultures containing 50% holo-Tf /50% apo-Tf compared with the other iron conditions (84.9% vs 47.1-67.2%). Comparable results were seen on culture days 13 and 17 (data not shown). Marrow culture studies of CEP patient 1 yielded similar data (supplemental Figure 1C). In contrast, the numbers of normal erythroid cells on culture day 10 progressively decreased when iron delivery was restricted (supplemental Figure 1D). (E) Expression of ALAS2 mRNA and protein in CEP cells during erythroid differentiation. With iron restriction (50% holo-Tf/ 50% apo-Tf; lane T), there is a slight reduction in ALAS2 mRNA (as normalized to β-actin) on culture day 10 (28.1 × 103 vs 31.6 × 103 in 100% holo-Tf; lane C; P = .21). More importantly, iron restriction dramatically reduces the amount of ALAS2 protein, confirming its primary effect on the translation of ALAS2 RNA to protein. On culture day 10, the intensity of ALAS2 (normalized to β-actin) in 50% holo-Tf/50% apo-Tf (lane T) is 43.1% of the intensity of ALAS2 in 100% holo-Tf (lane C). Globin protein is similar under both iron conditions. Also, CEP cells cultured in 50% holo-Tf/50% apo-Tf fully hemoglobinize as shown here by benzidine stains and in supplemental Figure 1E by Wright-Giemsa stains from culture day 13. These studies only used marrow from patient 2, as insufficient cells were available from patient 1.
Erythroid cell growth and maturation of CEP and normal bone marrow. (A) Flow cytometric profiles of control and CEP marrow cells cultured in standard iron-replete conditions (100% holo-Tf [holo-transferrin]; 8.54-9.52 μM iron). Although the erythroid cells from CEP patient 2 fully matured from stages I to IV (defined by the sequential gain of CD36 and then GlyA expression and the subsequent loss of CD36 expression), there were lower percentages of erythroid cells in stage III on day 10 (55.4% vs 64.4% in control cultures) and in stage IV on days 13 and 17 (33.1% and 73.0% vs 57.8% and 83.6%, respectively). Similar results were also seen in the studies of marrow cells from CEP patient 1 (supplemental Figure 1A). (B) Absolute numbers of erythroid cells in cultures containing 100% holo-Tf. Cultures of marrow cells from CEP patient 2 at day 10 have fewer cells than control cultures. This is mainly due to the decreased numbers of erythroid cells in stage III (19.0 × 105 vs 29.6 × 105), suggesting that CEP erythroid cells die when transiting to or in stage III. All cultures were initiated (day 0) with 2.0 × 106 bone marrow mononuclear cells. Similar results were also seen in marrow culture studies of CEP patient 1 (supplemental Figure 1B). (C) Total number of CEP erythroid cells on culture day 10 in media containing different ratios of holo-Tf to apo-Tf, as stated in Study design. The number of erythroid cells in cultures containing 100% holo-Tf was arbitrarily designated as 10 so that data from CEP patients 1 and 2 could be combined and both increases and decreases reported. Maximal erythroid growth occurred with 50% holo-Tf /50% apo-Tf. In this condition of partial iron restriction, there was a 2.5 ± 0.4-fold increase in the numbers of erythroid cells vs 0.9 ± 0.5- to 1.2 ± 0.1-fold changes in the other conditions. In contrast, the numbers of normal erythroid cells on culture day 10 did not change significantly when iron delivery is restricted. (D) Iron restriction also improves the erythroid maturation of CEP marrow cells. On culture day 10, a greater proportion of CEP patient 2’s cells were GlyA+ (stages III and IV) in the cultures containing 50% holo-Tf /50% apo-Tf compared with the other iron conditions (84.9% vs 47.1-67.2%). Comparable results were seen on culture days 13 and 17 (data not shown). Marrow culture studies of CEP patient 1 yielded similar data (supplemental Figure 1C). In contrast, the numbers of normal erythroid cells on culture day 10 progressively decreased when iron delivery was restricted (supplemental Figure 1D). (E) Expression of ALAS2 mRNA and protein in CEP cells during erythroid differentiation. With iron restriction (50% holo-Tf/ 50% apo-Tf; lane T), there is a slight reduction in ALAS2 mRNA (as normalized to β-actin) on culture day 10 (28.1 × 103 vs 31.6 × 103 in 100% holo-Tf; lane C; P = .21). More importantly, iron restriction dramatically reduces the amount of ALAS2 protein, confirming its primary effect on the translation of ALAS2 RNA to protein. On culture day 10, the intensity of ALAS2 (normalized to β-actin) in 50% holo-Tf/50% apo-Tf (lane T) is 43.1% of the intensity of ALAS2 in 100% holo-Tf (lane C). Globin protein is similar under both iron conditions. Also, CEP cells cultured in 50% holo-Tf/50% apo-Tf fully hemoglobinize as shown here by benzidine stains and in supplemental Figure 1E by Wright-Giemsa stains from culture day 13. These studies only used marrow from patient 2, as insufficient cells were available from patient 1.
To determine the effect of iron restriction on erythroid cell survival and differentiation, normal cells and CEP marrow cells were cultured in media containing different ratios of holo-Tf and apo-Tf media, as described in Study design. The greatest numbers of CEP cells were found in conditions of partial iron restriction (50% holo-Tf) vs higher or lower holo-Tf concentrations (Figure 2C). With 50% holo-Tf, more CEP cells also matured to stages III and IV (Figure 2D-E; supplemental Figure 1C, E). In contrast, the in vitro maturation of normal cells was hampered with reduced iron. The percentages of normal cells reaching stages III and IV at culture day 10 progressively decreased from 67.2% to 38.4% when available iron was reduced (supplemental Figure 1D), documenting their sensitivity to iron depletion. Iron restriction decreases the translation of ALAS2 mRNA, as shown in Figure 2E.
Discussion
Because CEP symptoms correlate with the degree of porphyrin excess,14 therapeutic interventions are often aimed at reducing levels of circulating porphyrins. Therapies such as hypertransfusion15,16 and oral charcoal17-20 have had limited success. Some mutations in UROS result in protein instability and degradation via the proteasome.21 Consistent with this, bortezomib has been shown to reduce porphyrin accumulation and photosensitivity in a murine model of CEP, although a trial in humans has yet to be initiated.22 Iron chelation has been used to ameliorate the accompanying iron overload that often develops in CEP patients.23 However, in the present case we initiated deferasirox to restrict iron availability. Our patient demonstrated a remarkable improvement in disease-related symptoms and more effective erythropoiesis with iron deficiency from both gastrointestinal losses and off-label use of deferasirox.
Iron restriction likely impedes heme synthesis upstream of UROS via decreasing ALAS2 mRNA translation (Figure 2E). Because the tricarboxylic acid cycle enzyme aconitase contains a 4Fe-4S cluster, it is also possible that decreased availability of succinyl coenzyme A, a key substrate for the rate-limiting step in heme production, may play a role. Erythroid cells obtained from the bone marrow of this CEP patient demonstrated improved growth and differentiation in conditions of relative iron deficiency. We propose that iron restriction might therapeutically benefit other patients with CEP and perhaps patients with other erythroid disorders involving the heme biosynthetic pathway.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank our patients and their family for allowing us to participate in their care and for providing biologic specimens for further study.
This work was supported in part by National Institutes of Health, National Cancer Institute training grant T32 CA009515 (to D.N.E.), National Heart, Lung, and Blood Institute training grant R01 HL31823 (to J.L.A.), National Institute of Diabetes and Digestive and Kidney Diseases training grant U54 DK083909 (to J.P.), and UL1 TR000423, which supports the University of Washington Clinical Research Center.
Authorship
Contribution: D.N.E. and J.L.A. wrote the manuscript; Z.Y. designed and performed laboratory studies and analyzed data; J.P. and J.L.A. designed experiments; and all authors edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janis L. Abkowitz, University of Washington, Department of Medicine, Division of Hematology, Box 357710, 1705 NE Pacific St, Seattle, WA 98195; e-mail: janabk@u.washington.edu.

![Figure 2. Erythroid cell growth and maturation of CEP and normal bone marrow. (A) Flow cytometric profiles of control and CEP marrow cells cultured in standard iron-replete conditions (100% holo-Tf [holo-transferrin]; 8.54-9.52 μM iron). Although the erythroid cells from CEP patient 2 fully matured from stages I to IV (defined by the sequential gain of CD36 and then GlyA expression and the subsequent loss of CD36 expression), there were lower percentages of erythroid cells in stage III on day 10 (55.4% vs 64.4% in control cultures) and in stage IV on days 13 and 17 (33.1% and 73.0% vs 57.8% and 83.6%, respectively). Similar results were also seen in the studies of marrow cells from CEP patient 1 (supplemental Figure 1A). (B) Absolute numbers of erythroid cells in cultures containing 100% holo-Tf. Cultures of marrow cells from CEP patient 2 at day 10 have fewer cells than control cultures. This is mainly due to the decreased numbers of erythroid cells in stage III (19.0 × 105 vs 29.6 × 105), suggesting that CEP erythroid cells die when transiting to or in stage III. All cultures were initiated (day 0) with 2.0 × 106 bone marrow mononuclear cells. Similar results were also seen in marrow culture studies of CEP patient 1 (supplemental Figure 1B). (C) Total number of CEP erythroid cells on culture day 10 in media containing different ratios of holo-Tf to apo-Tf, as stated in Study design. The number of erythroid cells in cultures containing 100% holo-Tf was arbitrarily designated as 10 so that data from CEP patients 1 and 2 could be combined and both increases and decreases reported. Maximal erythroid growth occurred with 50% holo-Tf /50% apo-Tf. In this condition of partial iron restriction, there was a 2.5 ± 0.4-fold increase in the numbers of erythroid cells vs 0.9 ± 0.5- to 1.2 ± 0.1-fold changes in the other conditions. In contrast, the numbers of normal erythroid cells on culture day 10 did not change significantly when iron delivery is restricted. (D) Iron restriction also improves the erythroid maturation of CEP marrow cells. On culture day 10, a greater proportion of CEP patient 2’s cells were GlyA+ (stages III and IV) in the cultures containing 50% holo-Tf /50% apo-Tf compared with the other iron conditions (84.9% vs 47.1-67.2%). Comparable results were seen on culture days 13 and 17 (data not shown). Marrow culture studies of CEP patient 1 yielded similar data (supplemental Figure 1C). In contrast, the numbers of normal erythroid cells on culture day 10 progressively decreased when iron delivery was restricted (supplemental Figure 1D). (E) Expression of ALAS2 mRNA and protein in CEP cells during erythroid differentiation. With iron restriction (50% holo-Tf/ 50% apo-Tf; lane T), there is a slight reduction in ALAS2 mRNA (as normalized to β-actin) on culture day 10 (28.1 × 103 vs 31.6 × 103 in 100% holo-Tf; lane C; P = .21). More importantly, iron restriction dramatically reduces the amount of ALAS2 protein, confirming its primary effect on the translation of ALAS2 RNA to protein. On culture day 10, the intensity of ALAS2 (normalized to β-actin) in 50% holo-Tf/50% apo-Tf (lane T) is 43.1% of the intensity of ALAS2 in 100% holo-Tf (lane C). Globin protein is similar under both iron conditions. Also, CEP cells cultured in 50% holo-Tf/50% apo-Tf fully hemoglobinize as shown here by benzidine stains and in supplemental Figure 1E by Wright-Giemsa stains from culture day 13. These studies only used marrow from patient 2, as insufficient cells were available from patient 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/2/10.1182_blood-2014-07-584664/4/m_257f2.jpeg?Expires=1769582513&Signature=YB3J0WTLyJw-m8wSsEigd0pXsOZNeUBwb~sEKXkl2nfBYMGglIA~Ir8d0Ml5HlBm9kk27AbRkVHSLK8qSSouL2SUcP4pMztroNvA46plDwLH8AUSCHLtx18Jy6jTa8-WkawJYSP2rqrIQqRHKS8d2f0qTdRWhdc8gt-lv2kldwSdkwb0goxVGIHw-SAjrsk8uNrKCXy8khjGS1FMQlWyY2pKUTRfDNltZvLBslCf4X8EKEGc7z9ZYbFctTVioK8U6k4yVm4kCGydjxM43GmLh5UQwStPTo31gIRhDFFDE5YTXhP7LggJWPXLIN~Cg38XlcclaK4BB0xS~EqWwcTx~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Erythroid cell growth and maturation of CEP and normal bone marrow. (A) Flow cytometric profiles of control and CEP marrow cells cultured in standard iron-replete conditions (100% holo-Tf [holo-transferrin]; 8.54-9.52 μM iron). Although the erythroid cells from CEP patient 2 fully matured from stages I to IV (defined by the sequential gain of CD36 and then GlyA expression and the subsequent loss of CD36 expression), there were lower percentages of erythroid cells in stage III on day 10 (55.4% vs 64.4% in control cultures) and in stage IV on days 13 and 17 (33.1% and 73.0% vs 57.8% and 83.6%, respectively). Similar results were also seen in the studies of marrow cells from CEP patient 1 (supplemental Figure 1A). (B) Absolute numbers of erythroid cells in cultures containing 100% holo-Tf. Cultures of marrow cells from CEP patient 2 at day 10 have fewer cells than control cultures. This is mainly due to the decreased numbers of erythroid cells in stage III (19.0 × 105 vs 29.6 × 105), suggesting that CEP erythroid cells die when transiting to or in stage III. All cultures were initiated (day 0) with 2.0 × 106 bone marrow mononuclear cells. Similar results were also seen in marrow culture studies of CEP patient 1 (supplemental Figure 1B). (C) Total number of CEP erythroid cells on culture day 10 in media containing different ratios of holo-Tf to apo-Tf, as stated in Study design. The number of erythroid cells in cultures containing 100% holo-Tf was arbitrarily designated as 10 so that data from CEP patients 1 and 2 could be combined and both increases and decreases reported. Maximal erythroid growth occurred with 50% holo-Tf /50% apo-Tf. In this condition of partial iron restriction, there was a 2.5 ± 0.4-fold increase in the numbers of erythroid cells vs 0.9 ± 0.5- to 1.2 ± 0.1-fold changes in the other conditions. In contrast, the numbers of normal erythroid cells on culture day 10 did not change significantly when iron delivery is restricted. (D) Iron restriction also improves the erythroid maturation of CEP marrow cells. On culture day 10, a greater proportion of CEP patient 2’s cells were GlyA+ (stages III and IV) in the cultures containing 50% holo-Tf /50% apo-Tf compared with the other iron conditions (84.9% vs 47.1-67.2%). Comparable results were seen on culture days 13 and 17 (data not shown). Marrow culture studies of CEP patient 1 yielded similar data (supplemental Figure 1C). In contrast, the numbers of normal erythroid cells on culture day 10 progressively decreased when iron delivery was restricted (supplemental Figure 1D). (E) Expression of ALAS2 mRNA and protein in CEP cells during erythroid differentiation. With iron restriction (50% holo-Tf/ 50% apo-Tf; lane T), there is a slight reduction in ALAS2 mRNA (as normalized to β-actin) on culture day 10 (28.1 × 103 vs 31.6 × 103 in 100% holo-Tf; lane C; P = .21). More importantly, iron restriction dramatically reduces the amount of ALAS2 protein, confirming its primary effect on the translation of ALAS2 RNA to protein. On culture day 10, the intensity of ALAS2 (normalized to β-actin) in 50% holo-Tf/50% apo-Tf (lane T) is 43.1% of the intensity of ALAS2 in 100% holo-Tf (lane C). Globin protein is similar under both iron conditions. Also, CEP cells cultured in 50% holo-Tf/50% apo-Tf fully hemoglobinize as shown here by benzidine stains and in supplemental Figure 1E by Wright-Giemsa stains from culture day 13. These studies only used marrow from patient 2, as insufficient cells were available from patient 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/2/10.1182_blood-2014-07-584664/4/m_257f2.jpeg?Expires=1769582514&Signature=AeFJymcb7iMsXrBZTBVX~KmgmUFnFrethMo2SpiCRf~M-ISdHn7T033xkHzEKqmqgIG8RrgE6pfkZG1UTIyxURrYB-5ZRiICjBn3WXbNJcxqY4NSYWmcopKDTELbdYxzZ3n5EIbwg~Gpr9dT42rw0pveLSe5CgkLUqdfa0VkCayA2moUMFY5bJ-yVCp9LUbMBuHtnAHewc3Mwk382i12di3ExJWvA9~Ot90uuo122yLwOJHAfdDNMjSqT4QK2wKbfB8dvPss-y0VZnAfw4Lkfj6Dg25K5J-NbYiCmVz5swIyKWUvyALLU~mmDNiDvzC2quhdU0rZ8XZKVQGNkNV~SQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)