Key Points
Coadministration of HU and an AKT2 inhibitor has beneficial effects on acute vaso-occlusive events and survival in SCD mice.
Abstract
Heterotypic cell-cell adhesion and aggregation mediate vaso-occlusive events in patients with sickle cell disease (SCD). Although hydroxyurea (HU), an inducer of fetal hemoglobin, is the main therapy for treatment of SCD, it is unclear whether it has immediate benefits in acute vaso-occlusive events in SCD patients. Using real-time fluorescence intravital microscopy, we demonstrated that short-term coadministration of HU and Akti XII, an AKT2 inhibitor, efficiently reduced neutrophil adhesion and platelet-neutrophil aggregation in venules of Berkeley (SCD) mice challenged with tumor necrosis factor α (TNF-α) or hypoxia/reoxygenation. Importantly, compared with HU or Akti XII treatment alone, short-term treatment with both agents significantly improved survival in those mice. We found that the level of plasma nitric oxide species was elevated by HU but not Akti XII, AKT2 phosphorylation levels in activated neutrophils and platelets were reduced by Akti XII but not HU, and the expression of endothelial E-selectin and intercellular adhesion molecule 1 was decreased by either agent. Our results suggest that short-term coadministration of HU and Akti XII has immediate benefits for acute vaso-occlusive events and survival in SCD mice exceeding those seen for single therapy.
Introduction
Sickle cell disease (SCD), an inherited blood disorder, results from a homozygous mutation at the 6th position (Glu6Val) of the β-globin chain (hemoglobin S [HbS]) or from compound heterozygous forms such as HbSC and HbS-β-thalassemia.1 HbS tends to polymerize in the deoxygenated state, and this leads to the sickling and hemolysis of red blood cells.2,3 Importantly, decreased nitric oxide (NO) bioavailability and increased oxidative stress contribute to the pathophysiology of SCD, including activation of endothelial cells (ECs), inflammation, and organ damage.4 Recurrent vaso-occlusive events, the hallmark of SCD, are mediated by adhesion and aggregation of red blood cells, leukocytes, and platelets on activated ECs5,6 and cause pain crises in SCD patients.7 Hydroxyurea (HU), the only drug approved by the US Food and Drug Administration for treatment of SCD, stimulates HbF production,8 serves as an NO donor,9,10 and inhibits tissue factor expression in leukocytes.11 Although the mechanism by which HU acts is still not fully understood, previous studies showed that treatment of SCD patients with HU is associated with the production of NO and increases HbF levels in an NO-dependent manner.9,10 Studies that use a humanized SCD (Berkeley) mouse model have consistently shown that short-term treatment with HU can have immediate benefits in vaso-occlusive events, independently of HbF production.12 The authors further demonstrated that combination therapy of HU and a phosphodiesterase 9 inhibitor efficiently inhibits acute vaso-occlusion in SCD mice.12
Intravital microscopic studies showed that neutrophil-platelet interactions on activated ECs are the major determinant of vascular occlusion during thromboinflammatory diseases in which inflammation is coupled to thrombosis.5,13,14 Although the mechanisms mediating neutrophil-platelet interactions remain poorly understood, previous studies showed that platelet and neutrophil AKT2 play critical roles in regulating platelet P-selectin exposure and activation of β2 integrins,5,15 thereby mediating neutrophil-EC and neutrophil-platelet interactions under inflammatory conditions. Importantly, we found that basal levels of AKT phosphorylation are significantly increased in neutrophils and platelets isolated from SCD patients compared with those in healthy donors and that short-term treatment with Akti XII, an AKT2-specific inhibitor, reduces neutrophil adhesion and platelet-neutrophil aggregation in venules of Berkeley mice, resulting in improved blood flow rates.5 Despite high homology (80%) in protein sequences of the 3 isoforms, our recent studies clearly indicated that AKT2 could be a dominant isoform regulating heterotypic cell-cell interactions and microvascular occlusion under inflammatory conditions.
In this study, we investigated whether short-term coadministration of HU and Akti XII has beneficial effects on acute vaso-occlusive events and survival in Berkeley mice challenged with tumor necrosis factor α (TNF-α) or hypoxia/reoxygenation.
Materials and methods
For a full description of all methods, see supplemental Methods, available on the Blood Web site.
Mice
Wild-type (WT) (C57BL/6, 6-week-old) hemizygous (Tg(Hu-miniLCRα1 GγAγδβS) Hba−/−Hbb+/–) and Berkeley sickle (Tg(Hu-miniLCRα1 GγAγδβS) Hba−/−Hbb−/−) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Berkeley mice were generated by transplantation of bone marrow cells isolated from Berkeley mice into lethally irradiated WT mice as described previously.16 The University of Illinois Institutional Animal Care and Use Committee approved all animal care and experimental procedures.
Intravital microscopy and survival times
Berkeley mice were injected with saline or HU 100 µg/g of body weight (g BW) via a tail vein and subsequently treated with intraperitoneal injection of TNF-α (500 ng) 3 hours prior to imaging. In other experiments, Berkeley mice were placed into an 8% O2 chamber for 3 hours to induce hypoxia, followed by 3 hours of reoxygenation in room air. The mice were treated with vehicle or HU (100 µg/g BW) at the beginning of reoxygenation via tail vein injection. Akti XII was administered through a jugular vein right before infusion of Dylight 488-conjugated anti-CD42c (platelet glycoprotein Ibβ) and Alexa Fluor 647-conjugated anti-Gr-1 (Ly-6G/Ly-6C) antibodies. Images were recorded and analyzed as described in supplemental Methods. During or after intravital microscopic studies, survival time for each mouse was measured. Each time point began at TNF-α injection (Figure 1A) or reoxygenation (supplemental Figure 1A) and ended when the mouse died or at 6 hours after TNF-α injection or reoxygenation.
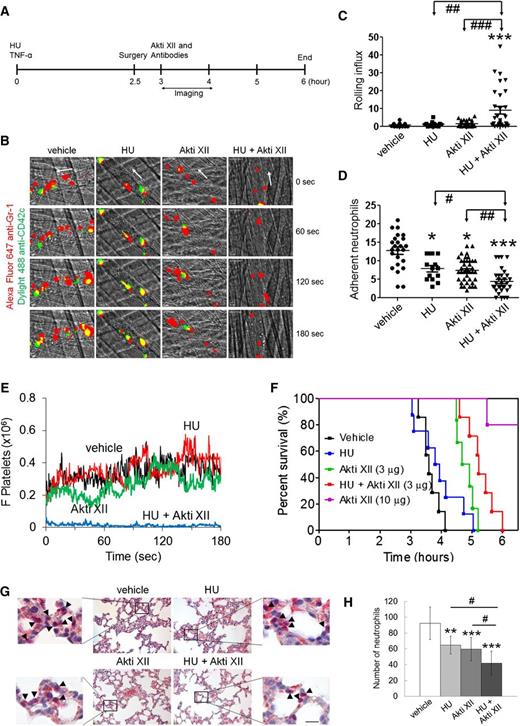
Coadministration of HU and Akti XII efficiently inhibits neutrophil adhesion and platelet-neutrophil aggregation in venules, improves survival, and impairs neutrophil transmigration into alveoli in TNF-α-challenged Berkeley mice. TNF-α was intraperitoneally injected into Berkeley mice to induce severe inflammatory conditions. Intravital microscopy was performed as described in supplemental Methods. Neutrophils and platelets were labeled by infusion of Alexa Fluor 647-conjugated anti-Gr-1 and Dylight 488-conjugated anti-CD42c antibodies. (A) Timeline for the treatment and surgery (jugular cannulation and cremaster muscle exposure) in Berkeley mice. White arrows show direction of blood flow. (B) Representative images of intravital captures at various time points. Time 0 was set as the image capture was initiated at each vessel. (C-D) Number of rolling and adherent neutrophils. (E) The integrated median fluorescence intensities of anti-CD42c antibodies (F platelets) were normalized to the number of adherent neutrophils and plotted as a function of time. Data were obtained from 45-57 venules in 6-8 mice per group. (F) Survival curves of Berkeley mice during or after intravital microscopy. (G-H) Representative images from histochemistry of lung sections. The number of transmigrated neutrophils (arrow heads) was quantified in the field of view (110 mm2). Data represent the mean ± standard deviation (n = 25-30 sections in 6-8 mice per group). Bar = 20 μm. The survival rate was assessed with Mantel-Cox log-rank test. *P < .05, **P < .01, and ***P < .001 vs vehicle control; analysis of variance (ANOVA) and Dunnett’s test. #P < .05, ##P < .01, and ###P < .001 between two groups; Student t test.
Coadministration of HU and Akti XII efficiently inhibits neutrophil adhesion and platelet-neutrophil aggregation in venules, improves survival, and impairs neutrophil transmigration into alveoli in TNF-α-challenged Berkeley mice. TNF-α was intraperitoneally injected into Berkeley mice to induce severe inflammatory conditions. Intravital microscopy was performed as described in supplemental Methods. Neutrophils and platelets were labeled by infusion of Alexa Fluor 647-conjugated anti-Gr-1 and Dylight 488-conjugated anti-CD42c antibodies. (A) Timeline for the treatment and surgery (jugular cannulation and cremaster muscle exposure) in Berkeley mice. White arrows show direction of blood flow. (B) Representative images of intravital captures at various time points. Time 0 was set as the image capture was initiated at each vessel. (C-D) Number of rolling and adherent neutrophils. (E) The integrated median fluorescence intensities of anti-CD42c antibodies (F platelets) were normalized to the number of adherent neutrophils and plotted as a function of time. Data were obtained from 45-57 venules in 6-8 mice per group. (F) Survival curves of Berkeley mice during or after intravital microscopy. (G-H) Representative images from histochemistry of lung sections. The number of transmigrated neutrophils (arrow heads) was quantified in the field of view (110 mm2). Data represent the mean ± standard deviation (n = 25-30 sections in 6-8 mice per group). Bar = 20 μm. The survival rate was assessed with Mantel-Cox log-rank test. *P < .05, **P < .01, and ***P < .001 vs vehicle control; analysis of variance (ANOVA) and Dunnett’s test. #P < .05, ##P < .01, and ###P < .001 between two groups; Student t test.
Statistics
Data were analyzed by using GraphPad Prism 5 software and by analysis of variance with Dunnett’s test, Student t test, and Mantle-Cox log-rank test. A P value less than .05 was considered significant.
Results
Coadministration of HU and Akti XII efficiently reduces neutrophil adhesion and platelet-neutrophil interactions in venules and prolongs survival times in TNF-α-challenged Berkeley mice
It was reported that treatment with HU at 100 µg/g BW partially inhibits neutrophil adhesion to the venules of TNF-α-challenged Berkeley mice.12 We recently demonstrated that platelets and neutrophils isolated from mice treated with 10 µg/g BW of Akti XII showed reduced phosphorylation of AKT2, but not AKT1 and AKT3, ex vivo and that 10 µg/g BW of Akti XII markedly decreased neutrophil adhesion and platelet-neutrophil aggregation in TNF-α-challenged Berkeley mice.5 By using a lower dose of Akti XII (3 µg/g BW), we performed intravital microscopy to determine the combined effect of coadministration of HU and Akti XII on vaso-occlusive events in Berkeley mice. The mice were treated with intravenous injection of a single dose of HU (100 µg/g BW) prior to intraperitoneal injection of TNF-α (Figure 1A). Surgical procedures were carried out at 2.5 hours after challenge with TNF-α followed by infusion of Akti XII. We observed that treatment with Akti XII or HU alone significantly reduced the number of adherent neutrophils with minimal effect on the rolling influx (Figure 1B-D and supplemental Videos 1-4). Compared with HU or Akti XII alone, combination treatment significantly increased the number of rolling neutrophils and further decreased neutrophil adhesion to ECs. As determined by the fluorescence intensities of anti-CD42c antibodies, Berkeley mice treated with HU or Akti XII exhibited no effect on platelet-neutrophil interactions (Figure 1E). In contrast, the mice treated with both agents showed a near complete inhibition of platelet-neutrophil interactions. These results suggest that coadministration of HU and Akti XII efficiently inhibits vaso-occlusive events in TNF-α-challenged Berkeley mice.
Intraperitoneal injection of TNF-α into Berkeley mice and subsequent surgical trauma lead to death within several hours of TNF-α injection as a result of acute vaso-occlusive events.12,16 We found that compared with the vehicle control, treatment with HU alone offered no increased survival, whereas treatment with Akti XII (3 µg/g BW) improved survival in the Berkeley mice (Figure 1F). Fifty percent of mice died at 3.6, 3.9, 4.8, and 5.2 hours after TNF-α injection in mice treated with vehicle, HU, Akti XII, and both HU and Akti XII, respectively (P = .0012 between HU and HU + Akti XII and P = .031 between Akti XII (3 μg/g BW) and HU + Akti XII). Most mice treated with 10 µg/g BW of Akti XII survived 6 hours after TNF-α injection, supporting our recent findings that AKT2 could be a novel target for treatment of thromboinflammatory disease.
Combination therapy efficiently inhibits neutrophil transmigration into the lung alveoli in TNF-α-challenged Berkeley mice
It was reported that increased vascular permeability may cause acute inflammation in the lungs of Berkeley mice.17 Thus, we performed histochemistry to measure neutrophil transmigration into the alveoli. The number of transmigrated neutrophils was decreased in HU- or Akti XII-treated Berkeley mice compared with the vehicle control (Figure 1G-H). Coadministration of HU and Akti XII further diminished neutrophil transmigration, suggesting that combination therapy has beneficial effects on pulmonary inflammation in TNF-α-challenged Berkeley mice, which may explain the improved survival after short-term treatment.
Coadministration of HU and Akti XII has beneficial effects on heterotypic cell-cell interactions and markedly improves survival in hypoxia/reoxygenation-challenged Berkeley mice
Previous studies showed that platelet-neutrophil aggregation is induced in blood isolated from Berkeley mice challenged with hypoxia (8% oxygen for 3 hours) and subsequent reoxygenation (room air for another 3 hours).18 By using this hypoxia/reoxygenation model, the same intravital microscopy was performed. Compared with TNF-α challenge, hypoxia/reoxygenation challenge showed increased rolling and decreased adhesion of neutrophils in venules of Berkeley mice (supplemental Figure 1B-D). Platelets adhered to neutrophils as a single cell and did not form thrombi (supplemental Figure 1E). The vehicle-treated mice died within 4.2 hours after reoxygenation (1.2 hours after surgery; supplemental Figure 1F). However, very few neutrophils transmigrated into the lung alveoli of hypoxia/reoxygenation-challenged Berkeley mice (data not shown). Compared with HU or Akti XII alone, coadministration of HU and Akti XII showed a significant increase in the rolling influx (supplemental Figure 1C). Strikingly, all mice treated with both agents survived until the end of the experiment (6 hours after reoxygenation; supplemental Figure 1F). Although the mechanisms mediating hypoxia/reoxygenation-induced death of Berkeley mice may be different from those in TNF-α-challenged mice, our results suggest that coadministration of HU and Akti XII has beneficial effects on vaso-occlusive events and survival in hypoxia/reoxygenation-challenged Berkeley mice.
The expression of E-selectin and intercellular adhesion molecule 1 (ICAM-1) is affected by treatment with HU or Akti XII
EC E-selectin and ICAM-1 are critical for neutrophil rolling and adhesion, respectively, during vascular inflammation.19 To determine the effect of HU and/or Akti XII treatment on the expression of those proteins, we performed immunohistochemistry using cremaster muscles taken from the mice after intravital microscopy. The expression of E-selectin and ICAM-1 was significantly decreased by HU or Akti XII treatment compared with the vehicle control (Figure 2A-D). E-selectin expression was further reduced in the mice treated with both HU and Akti XII, whereas no further reduction of ICAM-1 expression was observed. Because coadministration of HU and Akti XII is more beneficial than treatment with either of these alone in inhibiting cell-cell interactions and survival in TNF-α-challenged Berkeley mice, these results suggest that the function of blood cells, such as neutrophils and platelets, is also impaired by both agents.
The effects of HU and Akti XII on the expression of E-selectin and ICAM-1, plasma NOx levels, and AKT2 phosphorylation in TNF-α-challenged Berkeley mice. (A-D) Following intravital microscopy, the cremaster muscle was excised, fixed, and embedded in paraffin for immunohistochemistry. Sections of the muscle were labeled with rat anti-mouse E-selectin or ICAM-1 and then Dylight 488-labeled anti-rat immunoglobulin G antibodies, followed by incubation with phycoerythrin-labeled anti-PECAM-1 antibodies and a mounting reagent containing 4,6 diamidino-2-phenylindole. (A,C) Representative images of E-selectin or ICAM-1 and PECAM-1 staining. (B,D) The geometric mean fluorescence intensities (MFI) of E-selectin or ICAM-1 expression in venules of Berkeley mice. Data represent the mean ± standard deviation (SD) (n = 45-57 venules in 6-8 mice per group). (E) Following intravital microscopy, plasma NOx levels were measured as described in supplemental Methods. Data represent the mean ± SD (n = 6-8 mice per group). (F-I) Berkeley mice were treated with saline, HU, Akti XII, or both HU and Akti XII as described in supplemental Methods. Neutrophils and platelets were isolated and stimulated with fMLF and thrombin, respectively. Immunoblotting was performed by using equal amounts of protein (50 μg) followed by densitometry using Image J software. Representative blots (F,H) and quantitation of AKT2 phosphorylation after normalization to total AKT expression in neutrophils (G) and platelets (I). Data represent the mean ± standard error of the mean (n = 6 mice per group). *P < .05, **P < .01, and ***P < .001 vs vehicle control; ANOVA and Dunnett’s test. #P < .05 and ##P < .01 between two groups; Student t test.
The effects of HU and Akti XII on the expression of E-selectin and ICAM-1, plasma NOx levels, and AKT2 phosphorylation in TNF-α-challenged Berkeley mice. (A-D) Following intravital microscopy, the cremaster muscle was excised, fixed, and embedded in paraffin for immunohistochemistry. Sections of the muscle were labeled with rat anti-mouse E-selectin or ICAM-1 and then Dylight 488-labeled anti-rat immunoglobulin G antibodies, followed by incubation with phycoerythrin-labeled anti-PECAM-1 antibodies and a mounting reagent containing 4,6 diamidino-2-phenylindole. (A,C) Representative images of E-selectin or ICAM-1 and PECAM-1 staining. (B,D) The geometric mean fluorescence intensities (MFI) of E-selectin or ICAM-1 expression in venules of Berkeley mice. Data represent the mean ± standard deviation (SD) (n = 45-57 venules in 6-8 mice per group). (E) Following intravital microscopy, plasma NOx levels were measured as described in supplemental Methods. Data represent the mean ± SD (n = 6-8 mice per group). (F-I) Berkeley mice were treated with saline, HU, Akti XII, or both HU and Akti XII as described in supplemental Methods. Neutrophils and platelets were isolated and stimulated with fMLF and thrombin, respectively. Immunoblotting was performed by using equal amounts of protein (50 μg) followed by densitometry using Image J software. Representative blots (F,H) and quantitation of AKT2 phosphorylation after normalization to total AKT expression in neutrophils (G) and platelets (I). Data represent the mean ± standard error of the mean (n = 6 mice per group). *P < .05, **P < .01, and ***P < .001 vs vehicle control; ANOVA and Dunnett’s test. #P < .05 and ##P < .01 between two groups; Student t test.
The level of plasma NO metabolites is affected by short-term treatment with HU
Previous studies showed that the effect of oral administration of HU on the level of plasma NO metabolites could be different depending on SCD patients.10 Thus, we further measured the level of plasma nitrites and nitrates (NOx) in Berkeley mice after intravital microscopy. Blood was drawn immediately after the mice died or at the end of the experiment. We found that plasma NOx levels were increased by 2.4-fold in mice treated with HU alone or 2.1-fold in mice treated with both HU and Akti XII compared with the vehicle control (Figure 2E). There was no difference between groups treated with HU alone and groups treated with the combination. Treatment with Akti XII did not influence plasma NOx levels. Similar results were obtained in hypoxia/reoxygenation-challenged Berkeley mice (supplemental Figure 1G) in which the levels of circulating NOx were relatively higher than those in TNF-α-challenged Berkeley mice. In controls, treatment of WT (C57BL/6) and Hbb+/− (hemizygous) mice with HU alone significantly increased plasma NOx levels (supplemental Figure 2), suggesting that intravenous injection of HU (100 µg/g BW) serves as an NO donor in mice.
The level of cellular NO inhibits ICAM-1 expression in activated ECs
Because most actions of NO are intracellular and NO donors are known to inhibit ICAM-1 expression in activated ECs,20 we determined whether sodium nitroprusside (SNP), a well-known NO donor,21 affects ICAM-1 expression in TNF-α-activated ECs. Because of technical difficulties in isolating mouse vascular ECs, human umbilical vein ECs (HUVECs) were used in this study. Immunoblotting analysis showed that SNP treatment during TNF-α stimulation dose-dependently decreased ICAM-1 expression in HUVECs (supplemental Figure 3A-B). Complete inhibition was observed when HUVECs were stimulated with TNF-α in the presence of 10 μM SNP. These results support our speculation that NO produced by short-term treatment with HU may inhibit ICAM-1 expression in TNF-α-activated cremaster muscle ECs.
Treatment with Akti XII or both HU and Akti XII reduces the phosphorylation levels of neutrophil and platelet AKT2 ex vivo
We reported that AKT2 phosphorylation is significantly reduced in platelets and neutrophils isolated from WT mice treated with 10 μg/g BW of Akti XII.5 To further examine whether HU and a low concentration of Akti XII (3 μg/g BW) affect AKT2 phosphorylation in neutrophils and platelets ex vivo, Berkeley mice were treated with HU and Akti XII at 3 and 0.5 hours, respectively, prior to cell isolation. We found that the phosphorylation level of AKT2, but not AKT1, was significantly decreased in N-formyl-methionyl-leucyl-phenylalanine (fMLF)-stimulated neutrophils (Figure 2F-G) and thrombin-activated platelets (Figure 2H-I) isolated from the mice that received Akti XII alone or both HU and Akti XII compared with the cells isolated from vehicle- or HU-treated mice. There was no difference between mice treated with Akti XII alone and those treated with HU and Akti XII.
By using platelets and neutrophils isolated from Berkeley mice, we further examined whether SNP affects AKT2 phosphorylation during cell activation. We observed that SNP treatment at a concentration of 10 to 100 μM showed different effects on AKT2 phosphorylation in platelets and neutrophils: inhibition of AKT2 phosphorylation in thrombin-activated platelets, but enhancement of AKT2 phosphorylation in fMLF-stimulated neutrophils (supplemental Figure 3C-F). These results imply that dependent on the cellular level, NO may differentially regulate AKT2 phosphorylation in activated platelets and neutrophils. We found that treatment with 100 μM SNP significantly increased the cellular NOx levels in isolated platelets or neutrophils (supplemental Figure 3G-H). Pretreatment of platelets with 10 to 100 μM SNP compared with vehicle or 1 μM SNP elevated cellular NOx levels in a concentration-dependent manner following thrombin stimulation, whereas only the highest concentration (100 μM) of SNP increased the NOx levels in fMLF-stimulated neutrophils. Interestingly, platelet and neutrophil activation affected cellular NOx levels differently after pretreatment with 100 μM SNP; platelet activation significantly increased NOx levels, but neutrophil activation markedly decreased it. This may result from a large amount of reactive oxygen species produced from activated neutrophils, which can disrupt NO homeostasis.22
Discussion
Previous studies suggested that increased oxidative stress consumes NO, which decreases NO levels in Berkeley mice and that decreased NO bioavailability aggravates oxidative stress in SCD patients.23,24 It was reported that HU-induced HbF production is mediated by activation of soluble guanylyl cyclase in an NO-dependent manner.9 Transgenic Berkeley mice that expressed increased levels of HbF consistently exhibited a significant increase in the level of NO metabolites.23 Recent studies showed that the beneficial effects of intravenous injection of HU (100 μg/g BW) in TNF-α-challenged Berkeley mice was abolished when coadministered with an NO scavenger,12 suggesting that short-term treatment with HU serves as an NO donor. However, it is unclear whether such a high dose of HU can be used for intravenous injection in SCD patients because of the potential toxicity and whether short-term treatment of SCD patients with HU increases circulating NO levels. In this study, we found that, compared with the vehicle control, HU treatment significantly increased plasma NOx levels in Berkeley mice, which is likely to reduce oxidative stress. Furthermore, our ex vivo immunofluorescence microscopy showed that NO produced by HU treatment might inhibit ICAM-1 expression in TNF-α-activated cremaster muscle ECs, which was supported by our in vitro studies using SNP-treated HUVECs. Although HU treatment did not affect AKT2 phosphorylation in activated platelets and neutrophils ex vivo, our in vitro studies using SNP implied that NO might differentially regulate AKT2 phosphorylation in platelets and neutrophils. Future studies are required in SCD mice and patients to determine how much NO is produced by varying doses and administration times of HU and whether clinically approved NO donors have beneficial effects on acute vaso-occlusive events. How long-term treatment with HU affects plasma/cellular NO levels, which may differentially regulate the function of intravascular cells dependent on the cellular amount, should also be determined.
Studies with AKT2-null mice revealed that neutrophil AKT2 induces generation of reactive oxygen species by activating nicotinamide adenine dinucleotide phosphate oxidase 2 and mediates heterotypic cell-cell interactions under thromboinflammatory conditions and that platelet AKT2 is important for granular secretion and platelet aggregation.5,13,15,25 Therefore, inhibition of AKT2 in intravascular cells would be expected to attenuate oxidative stress and impair neutrophil adhesion and platelet-neutrophil interactions in vessels, thereby enhancing the beneficial effect of HU in Berkeley mice. Mechanistically, our results suggest that administration of either HU or Akti XII inhibits inflammatory conditions: short-term treatment with HU (100 μg/g BW) significantly increases plasma NO levels, short-term treatment with Akti XII (3 μg/g BW) decreases AKT2 phosphorylation in neutrophils and platelets without affecting plasma NO levels, and administration of either agent reduces surface expression of ICAM-1 and E-selectin on activated ECs. Thus, it is thought that the combined effects of both agents could result in immediate benefits: further inhibition of cell-cell interactions and significant improvement of survival in TNF-α- or hypoxia/reoxygenation-challenged Berkeley mice.
In addition to HU, numerous inhibitors have been tested in SCD mice and patients to reduce vaso-occlusive events. Some novel agents that are currently in clinical trials induce HbF production (decitabine and HQK-1001),26,27 inhibit the activation and adhesive function of neutrophils (GMI-1070)28 and platelets (Prasugrel),29 impair blood coagulation (Tinzaparin),30 and decrease oxidative stress (omega-3 fatty acids and N-acetyl cysteine).31,32 In particular, the randomized phase II study with GMI-1070 (Rivipansel), a pan selectin inhibitor, has shown that when given to SCD patients early in their hospitalization for treatment of vaso-occlusive events, it reduces the requirement for parenteral opioid analgesia and displays a trend toward decreased time to resolution as indicated by the patient’s readiness for discharge from hospital.28 Previous studies demonstrated that inhibition or deletion of AKT2 significantly decreases neutrophil and platelet activation, thereby reducing neutrophil-EC and platelet-neutrophil interactions and improving blood flow rates during vascular inflammation.5,15 Furthermore, this study reveals that coadministration of HU and Akti XII has beneficial effects on acute vaso-occlusive events and survival in TNF-α- or hypoxia/reoxygenation-challenged Berkeley mice. Because of the enrichment in insulin-responsive tissues, AKT2 is required to maintain normal glucose homeostasis.33 We found that one infusion of Akti XII at 10 to 30 μg/g BW into mice inhibits phosphorylation of AKT2, but not AKT1 and AKT3, in activated platelets and neutrophils5 and does not affect glucose tolerance (supplemental Figure 4). Although future studies are necessary to assess the potential toxicity of targeting AKT2, our results provide important evidence that an AKT2-specific inhibitor may be a short-term supplemental therapy for immediate benefits on acute vaso-occlusive events and survival in SCD patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alan Tseng and Drs Lewis Hsu, Robert Molokie, and Victor Gordeuk for their helpful comments. They also thank Dr Ramaswamy Ramchandran for allowing them to use his hypoxia chamber.
This work was supported in part by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL109439 to J.C.). J.L. and K.K. are recipients of American Heart Association postdoctoral fellowships.
Authorship
Contribution: A.B. designed and performed research, collected and analyzed data, and wrote the manuscript; J.L., K.K., and N.S. performed research; and J.C. initiated and designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaehyung Cho, 835 S Wolcott Ave, E403, Chicago, IL 60612; e-mail: thromres@uic.edu.