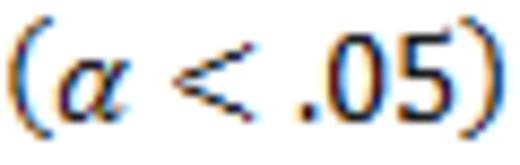Abstract
Background:
Eltrombopag (Epag) is an oral thrombopoietin receptor agonist used to increase platelet counts in patients with chronic immune thrombocytopenia (cITP). The maximum licensed Epag dose in cITP patients is 75 mg daily, yet some patients do not respond at this dose. Healthy individuals on escalated doses of eltrombopag (100-200 mg) demonstrated a dose dependent platelet response as did patients with thrombocytopenia secondary to chemotherapy. Doses of 100 mg and 150 mg have been approved for use in patients with hepatitis-C induced thrombocytopenia and aplastic anemia respectively. This is the final report of a double-blind, randomized controlled study to determine if Epag, administered at doses up to 150 mg daily, increases platelet counts in cITP patients who failed to respond to 75 mg.
Methods:
cITP patients³ 1 year old with platelet counts <50,000uL despite >3 weeks of 75mg of Epag daily), stratified by splenectomy status, were enrolled. Patients could continue stable doses of concomitant ITP medications. In the randomized blinded phase (Part 1), patients first received 75 mg daily of active Epag and 25 mg of study drug (Epag or placebo, 2:1). Every two weeks, study drug doses were increased in 25 mg increments to a maximum daily dose of 150 mg. After 8 weeks subjects were unblinded. If on active drug, they entered the open label phase (Part 2); if on placebo, they received open label Epag as per the study protocol escalating to a maximum dose of 150 mg. Two patients entered part 2 before 8 weeks because their counts were >100,000/uL in the blinded phase.
Data analysis was descriptive. Mann Whitney U test estimated p-values (α<.05) or platelet count differences between groups. Imputation of platelet counts allowed omitting falsely high or low platelet counts resulting from rescue therapy (e.g. IVIG), or counts in3 patients discontinuing early with good responses after 2, 2, and 4 weeks who thereby did not have counts at weeks 4, 6, and 8. Interim analysis was pre-specified and data is included on 33 of the planned total of 36 patients.
Results:
As of July 31, 35 patients consented; 26 completed ³8 weeks on study medication. Two patients are in part 1 and 7 dis-enrolled before completing 8 weeks of study drug: 5 failed screening and never received medication; one had bleeding and low counts on placebo; and one had pre-existing reticulin fibrosis 2-3+, discovered after study drug was dispensed but not administered.
The most common adverse event (AE) was minor bleeding. Two adults and 2 children developed transaminitis, which was life-threatening in 1 adult on 100mg. The 4 liver AEs occurred between 2 and 20 weeks of Epag dosing with 3/4 subjects on 150 mg daily; 2/4 discontinued Epag. No strokes or thromboembolic events were seen nor did any cataracts develop. Three patients underwent bone marrows: no grade 2-3 fibrosis was seen.
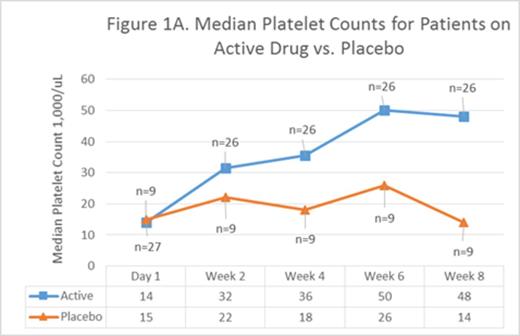
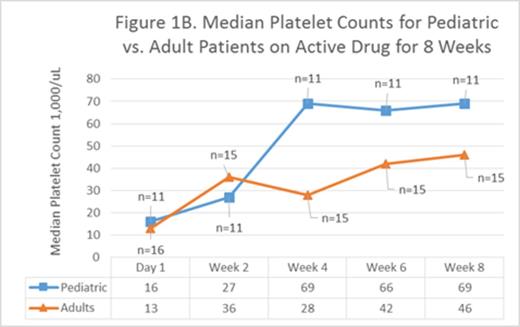
Platelet counts for patients on active drug vs placebo separated by week 2 (Figure 1A) and the difference steadily increased until week 8 (p<0.07) reflecting the thrombopoietic effects of increased dose Epag. Increased eltrombopag doses had greater effect in children (avg age 13) than adults (avg age 51) [Figure 1B]. This may reflect more rapid drug metabolism or relative insensitivity to the effects of Epag in children or both; this was first noted in PETIT and PETIT2.
Twenty-six patients entered the long-term open-label study. Three discontinued prior to 24 weeks because of transaminase elevation (1) and no response (2); 7 are not yet at 24 weeks; and 6 were also on other treatments, ie IVIG at increased intervals while on Epag, obscuring their responses. Ten patients were on open label medication for >24 weeks past the 8 weeks in part 1 up to 110 weeks. 72% of the long-term counts in these 10 patients were > 50,000/uL while 40% were > 100,000/uL. Six patients took 150 mg daily for >24 weeks while 4 reduced their doses, though not to <75mg daily.
Conclusions:
The results demonstrate that Eltrombopag at doses of 100-150mg daily can elevate platelet levels in most children and adults with cITP whose platelet counts were <50,000 on 75mg of Epag daily for >3 weeks. Children responded better to increased doses of Epag than did adults and high dose Epag can be used long term without development of cataracts, strokes or other thromboembolic events although an increased frequency of liver events, 12%, occurred. Although the 150 mg dose can be safely continued for many months, monitoring transaminases is essential.
Off Label Use: Increased doses of eltrombopag. McGuinn:Baxter: Research Funding; Octapharma: Membership on an entity's Board of Directors or advisory committees. Bussel:Momenta: Membership on an entity's Board of Directors or advisory committees; Genzyme: Research Funding; BiologicTx: Research Funding; Symphogen: Membership on an entity's Board of Directors or advisory committees; Cangene: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Protalex: Membership on an entity's Board of Directors or advisory committees; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Sysmex: Research Funding; Immunomedics: Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.