Abstract
Background: Pediatric hospital-acquired venous thromboembolism (HA-VTE) incidence is rising but remains relatively low overall, requiring risk stratification to reduce unnecessary thromboprophylaxis exposure. Large sample sizes are needed for prospective epidemiologic risk factor studies, necessitating collaboration.
Objectives: We formed the multi-institutional Children's Hospital-Acquired Thrombosis (CHAT) web-based registry via Research Electronic Data Capture (REDCap) to identify independent HA-VTE risk factors for future clinical risk score development.
Methods: This IRB-approved, retrospective chart review reveals HA-VTE risk factors from patients aged 0-21 years who developed diagnostically-validated VTE more than 48 hours after hospital admission, or after central venous line placement, at 3 pediatric hospitals from January 2012 - December 2014. We used descriptive statistics to summarize demographics, medical comorbidities and types of any applicable central lines for the initial 373 patients entered into the database, as well as characteristics of the VTEs themselves and associated laboratory testing. Further analysis is currently underway utilizing matched controls and logistic regression to identify specific odds ratios for independent risk factors.
Results: The median length of time to VTE diagnosis was 9 with interquartile range (IQR) of day 4-18, 35.3% of VTE occurred in a critical care unit, and 21% were incidentally found. The distribution of VTE included deep vein thromboses (DVT) of the arms/legs (81.1%) followed by cerebral sinus venous thrombosis (7.3%), pulmonary embolism (5.4%), DVT of the abdomen (4%), and intracardiac DVT (4%) with some overlap due to patients with multiple, separate, concurrent VTE events. Demographic characteristics of the initial 373 subjects revealed median age of 3.7 years (IQR of 0.4 years to 13.8 years) at VTE diagnosis and a slight male predominance (57.4%). 62.6% of patients had significant past medical history (Figure 1) and 8% were immobile at baseline.
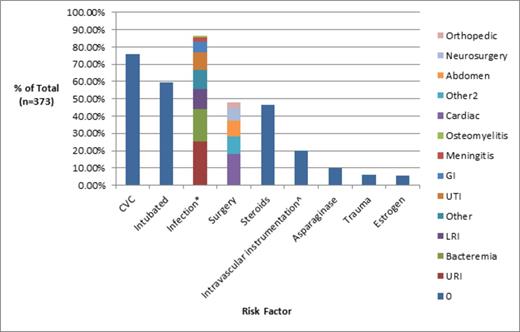
Evaluation of hospital course revealed a multitude of acquired putative risk factors for HA-VTE (Figure 2). 75.7% of VTE were associated with a central venous catheter (CVC). Of CVC-related VTE, 72% were in the same vein as CVC, 20% were in a vein which previously held a CVC, 3.6% surrounded the CVC tip, 2.9% occurred in a vein where CVC placement was attempted but unsuccessful. 59% of patients had at least one documented infection during hospitalization, 48% of patients had surgery, 5.5% of patients underwent trauma prior to admission, and 59.7% (n=221) of patients were intubated at some point during their admission with 86.9% (n=192) of those patients developing VTE after a minimum of 24 hours of mechanical ventilation. Laboratory testing of hospitalized patients revealed 51.2% of patients had a d-dimer level obtained at time of VTE and 97.8% of those patients had an elevated level. 44% of patients had at least one thrombophilia lab test ordered.
Conclusions: The initial CHAT database results demonstrate a slight male predisposition and multiple associated chronic medical illnesses and acquired hospital course co-morbidities, particularly CVCs which were involved in three-fourths of VTE events. Ongoing work includes incorporating additional institutions and utilizing control subjects to identify independent risk factors for the development of a risk score model. Long-term goals include prospective validation of the scoring system in a cohort of patients from pediatric centers not involved in development of the risk score with the ultimate plan of using the scoring system to stratify patients for future randomized clinical trials of risk-based prevention strategies to evaluate the safety and efficacy of this approach for reduction of pediatric HA-VTE incidence without unnecessary thromboprophylaxis exposure.
Prevalence of acquired risk factors. *Some patients with more than 1 documented infection. ^Procedures included: dialysis, plasmapheresis, cardiac catheterization, stent placement, coiling procedure.
Prevalence of acquired risk factors. *Some patients with more than 1 documented infection. ^Procedures included: dialysis, plasmapheresis, cardiac catheterization, stent placement, coiling procedure.
Young:Kedrion: Consultancy; Biogen Idec: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria; Bayer: Consultancy; Baxter: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.