Abstract
Introduction
Cancer is a risk factor for the development of venous thromboembolism (VTE). There is significant morbidity and mortality associated with VTE, and the treatment for these events can be painful, lengthy, and expensive. The development of DOACs has offered several new options for the treatment of VTE. Recent data suggested that the safety and effectiveness of DOACs in patients with cancer is equivalent to that of traditional oral therapies. The standard of care for the treatment of cancer-related VTE is a low molecular weight heparin (LMWH) formulation. If equivalent to LMWH, the use of DOACs in the treatment of cancer-related VTE would reduce the risk of VTE recurrence while potentially giving patients freedom from subcutaneous injections.
Methods
We performed a retrospective analysis of the electronic medical records (EMR) of adult patients with newly diagnosed or recurrent cancer-related VTE, who were treated with therapeutic dose of an anticoagulant for the index VTE and did not have other underlying thrombophilia or indication for long-term anticoagulation. Patients were treated at the benign hematology clinic during 2014. We screened a sample of 197 patients; 89 were selected in our final analysis according to the inclusion criteria. We used EMR to collect demographic data, laboratory values, details of cancer diagnosis, and details of VTE diagnosis. We documented patients who experienced recurrent VTE as well as those who experienced anticoagulant-associated clinically relevant bleeding. We used a multivariate analysis of this data to account for demographic, clinical, and tumor-related variables associated with this study. The primary outcome was the rate of recurrence of VTE after the initiation of anticoagulation. Secondary outcomes were the rate of anticoagulant-associated clinically relevant bleeding, as well as event-free survival for VTE recurrence.
Results
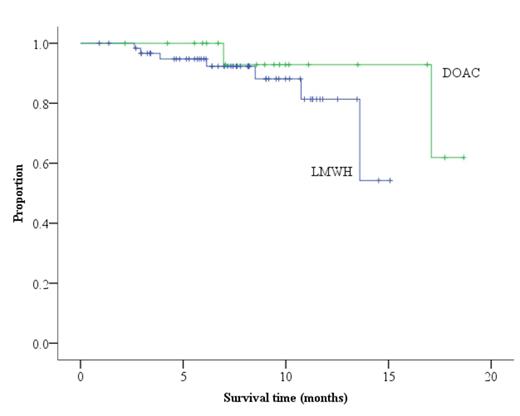
Among the 89 patients that were included in our final analysis, 63 (71%) were treated with subcutaneous formulations of low molecular weight heparin (LMWH), 20 (22%) were treated with DOACs, and 6 (7%) were treated with warfarin. The Eastern Cooperative Oncology Group (ECOG) score for these patients ranged from 0 to 3. The majority (69%) of VTE occurred in patients with stage III or IV disease. Almost all of the patients (91%) were diagnosed with VTE in the setting of active cancer; a small percentage of cases occurred in patients with a recent or remote history of malignancy. Patients had 36 different histological types of cancer, but the most common were invasive ductal carcinoma of the breast (21%), lymphoma (9%), and adenocarcinoma of the colon or rectum (6%). Most of the patients (91%) were free of primary or metastatic disease of the central nervous system, but nearly 1 in 5 (17%) had evidence of hepatic metastases at the time of the index event. The most common index event was pulmonary embolism (53%) followed by catheter-associated deep venous thrombosis (DVT) (28%) and proximal lower limb DVT (12%). Recurrence of VTE occurred in 10.0% of patients receiving DOACs and 11.1% of patients receiving LMWH (P=NS). When VTE recurrence occurred, it was most likely in the form of pulmonary embolism (44%), splanchnic vein thrombosis (22%), or proximal lower limb DVT (11%). Clinically relevant bleeding was slightly higher with the use of DOACs (20%) versus LMWH (17%). Interestingly, major bleeding occurred more often with the use of DOACs (15%) than with LMWH (6.3%), although clinically relevant non-major bleeding was more common in patients receiving LMWH (11%) than in those receiving DOACs (5%). There was no mortality attributed to bleeding complications. The VTE recurrence-free survival rates were not statistically different (P=0.222) among patients treated with LMWH versus DOACs (Figure 1)
Conclusion
Cancer-associated VTE is associated with significant morbidity and mortality. Recurrence of VTE is not uncommon, and caution must be exercised throughout the treatment of VTE due to the significant anticoagulant-associated hemorrhagic risks. LMWHs are effective for the treatment of cancer-related VTE, but injections are cumbersome and painful for many patients. Our analysis suggests that there is no significant difference in the rate of VTE recurrence when using oral DOACs versus subcutaneous LMWH. DOACs appeared to be at greater risk of major bleeding.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.