Abstract
Immunomodulation following transfusion of cellular blood products presents as a generalized immunosuppression and/or production of alloantibody. The immunological effects of transfusion in immunocompetent subjects were evaluated in patients undergoing cardiac surgery with cardio-pulmonary by-pass.
Subjects were consented for this IRB-approved study and were randomized to receive unmodified (STD) red blood cells (rbc), leukoreduced (LR) rbc or LR rbc that were g-irradiated (g-I). All platelet transfusions were collected LR and they were also g-I for the third arm. Patients were transfused only when clinically indicated. Subjects receiving no transfusions during the study served as a fourth control arm. Peripheral blood samples collected prior to surgery were controls for samples collected after surgery (day 10 +/- 4 and day 30 +/-5).
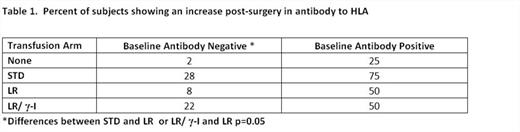
HLA antibody production was evaluated in 245 subjects using single-antigen multiarray assays for antibody to both HLA class I and class II, a summary of results in shown in Table 1. For subjects with HLA antibody in their pre-surgery sample, these assays allowed evaluation of change in antibody specificity or levels. For these pre-sensitized subjects, there was no significant difference in HLA antibody production between the transfused study arms: even those not transfused showed an increase, most likely due to activation of memory responses. For subjects without HLA antibody in their pre-surgery sample, recipients of LR products showed little increase supporting prior studies showing the efficacy of LR in reducing antibody. However the protection afforded by LR was abrogated by g-I. This was an unexpected finding.
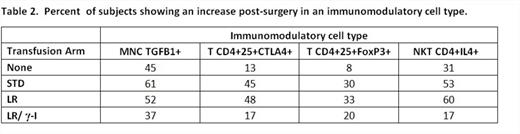
We evaluated cellular and cytokine mediators of immunosuppression in the first 139 consecutive subjects of the 245 subjects enrolled in this study. This part of the study was powered for hypothesis generation, not statistical significance. Mononuclear cells (MNC) were isolated from each sample, frozen, and all samples from a subject assayed together. We assayed these cells for production of TGFB1 using ELISPOT. We used flow cytometry phenotyping for markers of CD4+25+ T regulatory cells (CTLA4, FoxP3) and for intracellular cytokines for CD4+ and CD4- NKT cells (IFNG, IL-4, IL-10). As shown in Table 2, recipients of standard rbc showed an increase (following transfusion and surgery) in TGFB1 producing mononuclear cells with a lower increase in recipients of LR rbc and those who were not transfused, but further reduced in the group receiving g-I LR products. Recipients of standard rbc or of LR rbc showed an increase of T lymphocytes expressing FoxP3 or CTLA4, and of CD4+ NKT cells producing IL-4. In contrast, subjects whose LR products were g-I showed a markedly lower increase in all three measures. These data identify an increase in mediators of immunosuppression in recipients of standard rbc and LR rbc that was reduced in recipients of g-I and LR rbc and platelets. Surgery and bypass alone increased the numbers of TGFB1+ MNC and IL4+ NKT cells suggesting a role for the innate immune system in immunosuppression following transfusion.
This study is the first to exam the immunomodulatory effects of transfusion by combining measurement of immunosuppression in ex vivo surrogate assays and measurement of HLA antibody production inclusive of pre-sensitized subjects. Our observations of differences in responses to blood products prepared with g-I plus LR compared to LR alone were unexpected and require confirmation. It suggests that the reduction in immunosuppressive cells and cytokines in the recipients of g-I and LR products contributed to the increased allosensitization we observed in that arm compared to recipients of LR products. It may be possible to manipulate immunomodulation after transfusion to reach a desired goal for specific patient populations.
Slichter:Department of Defense: Research Funding; Terumo BCT: Research Funding; Cerus Corporation: Research Funding; Cellphire, Inc.: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.