Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive leukemia predominantly found in children that has a worse prognosis than other forms of pediatric leukemia with few options for relapsed disease. These issues lead to a need for new targeted therapies. Many of the genes critical for normal T-cell differentiation are aberrantly expressed when patients develop T-ALL, and this knowledge can serve as the basis for rationale design of targeted therapies. In this list are DNA methyltransferase 3A (DNMT3A), a protein that is responsible for de novo DNA methylation, critical for the function of hematopoietic stem cells (HSCs), and NOTCH1, a protein involved in T-cell signaling and regulating cell fate decisions. Patients that with DNMT3A loss-of-functions mutations driving T-ALL have a worse prognosis than their counterparts with normal DNMT3A function. Previous attempts to directly target the action of NOTCH1 have not led to success, specifically gamma secretase inhibitors, so we aim to define the epigenetic regulation of this pathway and the co-operation between DNMT3A and NOTCH1 mutations may open the door for new therapies in patients with the poorest outcomes.
We show here that ablation of Dnmt3a in murine HSCs using Mx1-Cre (Mx1-CRE:Dnmt3afl/fl) results in abnormal T-cell development, leading to an accumulation of progenitors at the double negative 2 (DN2) stage (CD4-, CD8-, c-Kit+, CD44+, CD25+) in a proportion of mice (but not all). In affected mice, although there is a significant accumulation of these progenitors, the downstream population of mature T-cells is essentially unchanged compared to wild-type mice. This developmental arrest is associated with increased expression of the Notch1 signaling pathway. Initially suspected as the mechanism given the native action of Dnmt3a, was differential DNA methylation of Notch1 and its downstream targets such as Hes1 and c-Myc, however, through performed bisulfite sequencing of the promoters for those genes and discovered that there was no difference in DNA methylation between wild-type and Dnmt3a-null thymic progenitor cells. The cellular difference underlying accumulation of this Dnmt3a-null thymic progenitor population appears to be increased resistance to apoptosis, and molecular studies are ongoing to explore the molecular mechanism behind this.
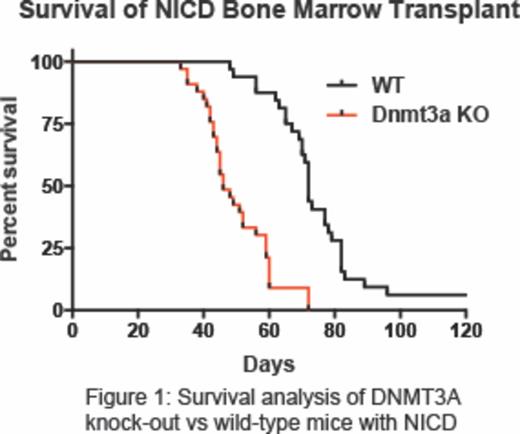
The observations that thymic progenitor cells lacking Dnmt3a have modified expression of Notch1, and mutations of both DNMT3A and NOTCH1 have been found in T-ALL patient samples, indicates potential co-operation between these two pathways in T-ALL oncogenesis. To test the biological co-operation between these pathways in vivo, we introduced activated Notch1 intracellular domain (NICD) into wild-type and Dnmt3a-null hematopoietic progenitor cells and assessed the latency to T-ALL by bone marrow transplantation. Introducing NICD into a Dnmt3a-null background significantly accelerated T-ALL oncogenesis (median survival wild-type NICD = 72-days, median survival Dnmt3a-null NICD = 46-days, p < 0.001, Figure 1). To explore the molecular differences leading to enhanced T-ALL pathogenesis in a Dnmt3a-null background, we performed RNA-Seq on T-ALL blasts and found that murine leukemia samples with the combination of Dnmt3a loss-of-function and constitutive Notch1 activation show increased in Jak/Stat pathway signaling compared to T-ALL blasts with native Dnmt3a and normal double positive T-cells (CD8+, CD4+).
We hypothesize that Jak/Stat pathway inhibitors could improve outcomes for T-ALL patients with combination DNMT3A loss-of-function and activating NOTCH1 mutations. Ruxolitinib is a well-known JAK1/JAK2 inhibitor used in the treatment of myelofibrosis where it has produced robust results. Given the alterations in Jak/Stat signaling, we plan to induce leukemia by elimination of Dnmt3a using the Cre based model, followed by viral introduction of the Notch1 mutant and then treat mice with ruxolitinib and compare survival. It is our hypothesis that the drug will increase latency to leukemia in this quick to develop murine model. Using developed drugs to target new pathways in T-ALL has the potential to offer new strategies to patients with difficult to treat relapsed disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.