Abstract
Background: The evolution of BCR-ABL1 tyrosine kinase inhibitors (TKIs), from imatinib to newer agents such as nilotinib, has led to progressively lower levels of disease burden in patients with CML receiving TKI therapy. Currently, real-time quantitative PCR (RQ-PCR) is the most commonly used test for assessing MR in patients with CML and is able to detect BCR-ABL1 transcript levels down to MR4.0 to MR4.5 on the IS. It has been reported that dPCR can detect BCR-ABL1 with greater sensitivity and precision than RQ-PCR. In addition, unlike RQ-PCR, dPCR is able to perform absolute copy number quantification without the use of calibrators; thus, it should be free from calibrator biases. To date, validation of dPCR BCR-ABL1 assays with multiple control genes and standardization to the World Health Organization (WHO) IS reference material have not been reported. Standardization to the IS is critically important to enable appropriate assessment of patient response to therapy and to establish a standard of care. In this study, we describe the development, validation, and IS standardization of a sensitive BCR-ABL1 dPCR assay using 3 different control genes. We also tested the comparability of 3 different dPCR platforms.
Methods: Four experiments were included in this study: (1) Three BCR-ABL1 dPCR assays were developed using ABL1, BCR, and GUS as control genes on the Bio-Rad dPCR platform. The ABL1 assay was standardized to the IS using the WHO Reference Panel. The BCR and GUS IS alignments are currently in process. All 3 assays were validated for linearity, precision, accuracy, limit of detection, and limit of blank, following in vitro diagnostics industry best practices and Clinical and Laboratory Standards Institute (CLSI) guidelines. (2) Multiple BCR-ABL1 and ABL1 primer/probe sets were tested on both RQ-PCR and dPCR for the selection of the best primer/probe design. (3) BCR-ABL1 and ABL1 quantifications were compared between 3 dPCR platforms: Bio-Rad QX200, RainDance RainDrop System, and Applied Biosystems QuantStudio3D. (4) Sensitivity of the BCR-ABL1 dPCR assay will be compared in CML PAXgene vs K2EDTA blood tube samples to assess the potential impact of blood-collection tube type on deep MR measurement in patients with CML.
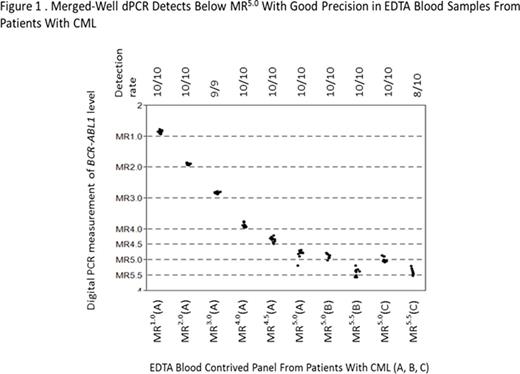
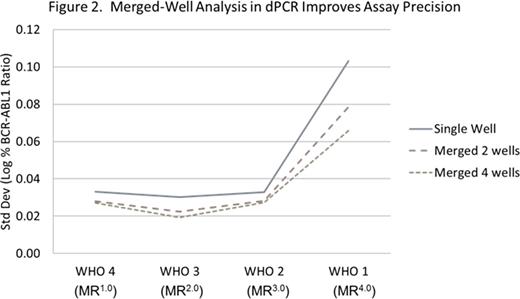
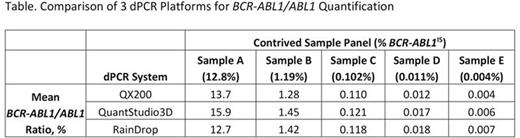
Results: (1) The BCR-ABL1/ABL1 dPCR assay WHO IS alignment resulted in a conversion factor of 1. Using the WHO IS-calibrated BCR-ABL1/ABL dPCR assay, MR in blood samples from patients with CML were quantified with sensitivity beyond MR4.5 to levels between MR5.0 and MR5.5 (Figure 1). Combining multiple dPCR wells, which enables analysis of thousands to millions of reactions together, improved the sensitivity and precision of the assay (Figure 2). (2) Evaluation of 10 BCR-ABL1 and 9 ABL1 primer/probe sets using the Bio-Rad dPCR platform resulted in < 2-fold differences in copy number detection compared with the 16- to 32-fold difference using RQ-PCR. This is likely due to dPCR measurement being an endpoint PCR reaction. (3) Less than 2-fold differences in both detected copy number and BCR-ABL1/ABL1 ratios were observed using 1 selected primer/probe set when a contrived sample panel representing key BCR-ABL1 levels from 10% to 0.0032% IS was tested across 3 different dPCR platforms, suggesting good cross-platform robustness of dPCR (Table). (4) The PAXgene vs K2EDTA blood tube comparison study is ongoing; data will be presented upon completion of the study.
Conclusion: Three dPCR BCR-ABL1 assays were developed. The BCR-ABL1 dPCR assay demonstrated detection capability at levels below MR4.5, down to MR5.0 to MR5.5 in contrived samples from patients with CML. This increased sensitivity relative to RQ-PCR may aid future comparisons of deep MR rates across different CML therapies. dPCR assay performance is also more robust against primer/probe design changes than RQ-PCR, thus requiring less assay optimization. Our results demonstrate that dPCR offers robust BCR-ABL1 quantification on the WHO IS across commonly used control genes without requiring calibrators or extensive assay optimization.
Huang:Novartis: Employment. Salcius:Novartis: Employment, Equity Ownership. Wong:Novartis: Employment, Equity Ownership. Wong:Novartis: Employment. Wang:Novartis: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract