Abstract
Introduction
Relapse in the central nervous system is an uncommon complication of diffuse large B cell lymphoma (DLBCL) associated with a poor prognosis. The addition of rituximab to chemotherapy has improved outcomes in patients with DLBCL but its effect on the outcome of patients with CNS relapse is not well characterized. Here we present the natural history of patients with CNS relapse in a large cohort of patients with DLBCL who were treated in the immunochemotherapy (IC) era.
Methods
Newly diagnosed patients with DLBCL or primary mediastinal B-cell lymphoma (PMBCL) and treated with primary anthracycline based IC were prospectively enrolled on the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER) within 9 months of diagnosis and followed for relapse, retreatment, and death. Clinical management at diagnosis and subsequent therapies were per treating physician. Patients with documented CNS involvement at diagnosis, primary CNS lymphoma, or post-transplant lymphoproliferative disorder at diagnosis were excluded. All CNS relapse and retreatment details were verified by medical record review.
Results
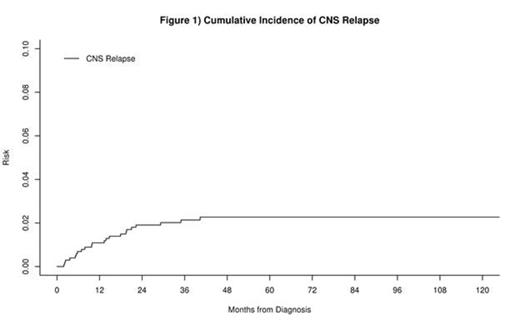
1017 patients with newly diagnosed DLBCL or PMBCL, no documented CNS disease at diagnosis, and treated with IC were enrolled in the MER between 2002 and 2012. At a median follow-up of 59 months (range 1-148), 36 patients had CNS relapse. The cumulative incidence of CNS relapse was 3.1% (95% CI: 2.2%-4.4%) at 2 years and 3.7% (95% CI: 2.7%-5.0%) at 5 years after diagnosis. CNS involvement was identified after first line IC in 25 patients, and after salvage therapy in 11 patients. 22 patients had an isolated CNS relapse, while 14 had both systemic and CNS disease at relapse. The incidence of isolated CNS relapse at two years from diagnosis was 1.9% (95% CI: 1.2%-3.0%, figure 1). CNS involvement was parenchymal in 22 patients, leptomeningeal in 11, and parenchymal and leptomeningeal in 3 patients.
At diagnosis, this subset of 36 patients had a median age of 61 years (range 20-86); 25 (69%) were male. IPI was 0-1 in 6 patients, 2 in 13 patients, 3 in 11 patients and 4-5 in 6 patients. Cell of origin per Hans algorithm was available in 17 patients, 8 were GCB and 9 were non-GCB. Extranodal sites of disease at diagnosis included testicular (2), renal (5), bone (10) and bone marrow (6). 7 patients had no sites of extranodal disease. LDH was elevated in 71%. The German High-Grade Non-Hodgkin Lymphoma Study Group CNS risk score was low risk in 6 patients, intermediate risk in 22 patients, and high risk in 8 patients. 6 patients received CNS prophylaxis with their initial immunochemotherapy, including 3 of the 21 patients who subsequently developed isolated CNS disease.
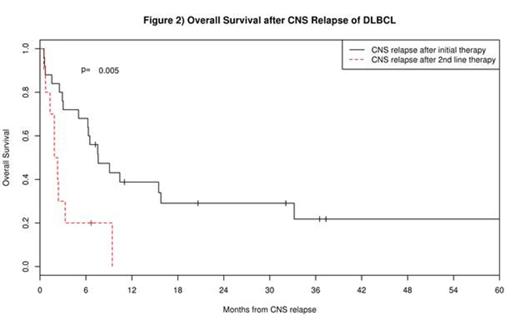
First line therapy after CNS relapse included high dose methotrexate based chemotherapy in 22 patients and other systemic regimens in 6, with intrathecal (IT) chemotherapy in 5. Overall survival in patients after CNS relapse was poor with a median survival of 6.3 months (95% CI: 2.9-15.5) and 12-month survival rate of 29% (95% CI: 17%-49%). Patients who had CNS relapse following initial IC had better subsequent survival (median OS = 7.6 months) compared to patients with first presentation of CNS relapse following salvage therapy (OS = 2.1 months, p=0.005, see figure 2). 10 patients (28%) proceeded to SCT after CNS relapse, of which 6 remain alive at a median follow-up of 39 months. There were no differences in post-CNS relapse survival by age, sex, CNS risk score, cell of origin, extranodal site involvement at diagnosis, or site of CNS relapse (all p>0.10), though power was limited due to small numbers.
Conclusions
The incidence of CNS relapse in DLBCL is approximately 4% in the immunochemotherapy era. The vast majority of CNS relapses occur within 2 years of diagnosis. Isolated relapse in patients without CNS involvement at diagnosis is a relatively rare event with an incidence of approximately 2% at 2 years of diagnosis. Outcomes remain poor, and standard clinical variables do not predict survival after CNS relapse. Novel therapeutic approaches are needed in this population, with consideration to autologous SCT, which produces durable remission in a subset of patients.
Maurer:Kite Pharma: Research Funding. Farooq:Kite Pharma: Research Funding. Thompson:Kite Pharma: Research Funding. Ansell:Bristol-Myers Squibb: Research Funding; Celldex: Research Funding. Cerhan:Kite Pharma: Research Funding. Link:Genentech: Consultancy, Research Funding; Kite Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.