Abstract
Few studies have compared treatment outcomes and disease complications between classical and variant hairy cell leukemia (HCL).
We reviewed records of patients (pts) with HCL treated at Memorial Sloan Kettering Cancer Center between 1983 and 2013 and identified 331 pts. To reduce bias we limited analysis to the 183 pts who were reviewed and treated at MSKCC within 3 months of diagnosis (table 1).
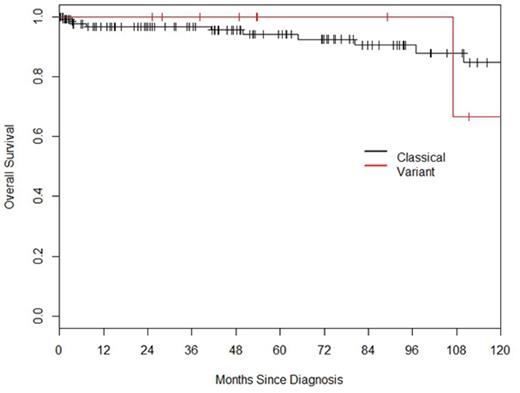
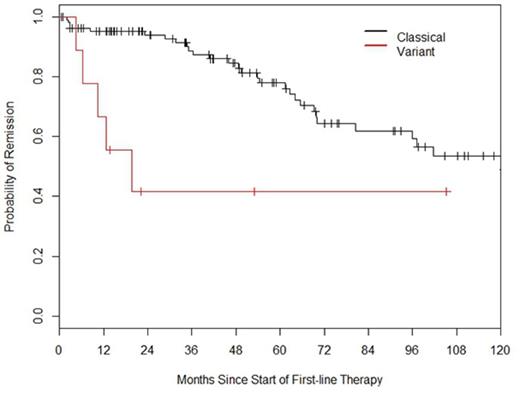
The median follow-up was 46.8 months. Median overall survival (OS) for the entire cohort was not reached and 5 and 10 year OS was 94% and 83% respectively. Median OS for classical and variant HCL was not reached in either group (Fig 1) while 5 and 10 year OS appeared equal. The time to next treatment (TNT) following initial therapy was longer for classical HCL (Fig 2). Pts with classical HCL were also more likely to achieve remission with first therapy and required fewer individual lines of therapy (Table 1). Cladribine was used first line in 122 pts and resulted in a median TNT of 138 months (97.1-NA), pentostatin was used in 9 pts and resulted in similar remission duration as cladribine with TNT of 81 months (80.5-NA) (p=0.82). 5 pts had abnormal cytogenetics at diagnosis and this did not influence OS when compared to the 62 pts who had a normal karyotype with estimated 5 year OS of 100% and 97%, respectively.
31 pts required treatment for disease relapse. The median time to 3rd therapy was not reached however 72% and 52% of all pts were estimated to require a 3rd treatment at 5 and 10 years, respectively. Cladribine was used to treat 1st relapse in 18 pts while 5 were treated with the combination of cladribine and rituximab. This resulted in a median TNT of 66.3 (37.2-NA) months in the cladribine group, median TNT was not reached for the combination group. We found that initial treatment of HCL with cladribine appeared to result in a longer disease remission when compared with the second treatment with a median TNT of 138 and 66 months respectively. 22 pts died during follow up, with 2 deaths due to HCL, 6 due to secondary malignancy, the remainder were unknown.
27 Secondary cancers were identified the most common were non-melanoma skin cancer (5), prostate cancer (5), melanoma (4) and other lymphoproliferative disorders (4). The most common reasons for needing retreatment varied depending on disease type with the recurrence of cytopenias accounting for 23/25 pts with classical HCL and only 1 with variant HCL. Symptomatic splenomegaly prompted re-treatment in 4/5 with variant and 3/25 classical HCL. B symptoms were uncommon occurring in 1 pt with classical and 1 with variant disease at relapse. The major complication of 1st therapy was febrile neutropenia necessitating admission for intravenous antibiotics, which occurred in 40/139 pts. There were no mortalities due to bacterial sepsis following 1st therapy.
We conclude that OS of pts with HCL variant is equal to that of classical disease; however patients with classical HCL have a far longer duration of first remission and a greater chance of complete remission. Patients with variant HCL have different clinical features at relapse and appear to require more lines of therapy to maintain disease control. We found that responses to cladribine following first treatment appear longer than for second treatment. The combination of cladribine and rituximab may result in a longer second remission. We did not find a higher incidence of secondary malignancy in patients with variant HCL.
Comparison of patients with classical and variant HCL.
| . | Classical HCL (N=146) . | Variant HCL (N=10) . | . |
|---|---|---|---|
| Median age (range) | 52 (27-84) | 67 (39-78) | P=0.005 |
| Men (%) | 114 (78%) | 6 (60%) | |
| Median WBC at diagnosis | 3.7x10^9/L | 9.7x10^9/L | |
| CD25 expression | 146/146 | 0/10 | |
| BRAF* (V600E mutated/ assessed) | 3/6 | 1/2 | |
| Number needing therapy | 109 | 10 | |
| Indication for re-treatment | 25 | 5 | |
| Cytopenia | 23/25 | 1/5 | |
| splenomegaly | 3/25 | 4/25 | |
| Median OS | Not reached | Not reached | |
| 5 year OS | 94% | 100% | |
| 10 year OS | 84% | 67% | |
| TNT following 1st treatment (months) | 120 | 20 | P=0.002 |
| CR to 1st therapy | 87/109 | 5/10 | |
| Median number of therapies (range) | 1 (0-7) | 4 (2-7) | |
| Splenectomy | 1 | 3 | |
| Secondary cancers | 27 | 0 | |
| Deaths | 15 | 1 |
| . | Classical HCL (N=146) . | Variant HCL (N=10) . | . |
|---|---|---|---|
| Median age (range) | 52 (27-84) | 67 (39-78) | P=0.005 |
| Men (%) | 114 (78%) | 6 (60%) | |
| Median WBC at diagnosis | 3.7x10^9/L | 9.7x10^9/L | |
| CD25 expression | 146/146 | 0/10 | |
| BRAF* (V600E mutated/ assessed) | 3/6 | 1/2 | |
| Number needing therapy | 109 | 10 | |
| Indication for re-treatment | 25 | 5 | |
| Cytopenia | 23/25 | 1/5 | |
| splenomegaly | 3/25 | 4/25 | |
| Median OS | Not reached | Not reached | |
| 5 year OS | 94% | 100% | |
| 10 year OS | 84% | 67% | |
| TNT following 1st treatment (months) | 120 | 20 | P=0.002 |
| CR to 1st therapy | 87/109 | 5/10 | |
| Median number of therapies (range) | 1 (0-7) | 4 (2-7) | |
| Splenectomy | 1 | 3 | |
| Secondary cancers | 27 | 0 | |
| Deaths | 15 | 1 |
*BRAF mutation was infrequently assessed as we analyzed patients up to 2013.
OS for classical and variant HCL is equal despite the shorter remission duration and more frequent need for re-treatment in HCL variant.
OS for classical and variant HCL is equal despite the shorter remission duration and more frequent need for re-treatment in HCL variant.
Remission duration following first therapy is significantly longer in patients with classical than variant HCL.
Remission duration following first therapy is significantly longer in patients with classical than variant HCL.
Park:Actinium Pharmaceuticals, Inc.: Research Funding; Juno Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.