Abstract
DI-Leu16-IL2 immunocytokine is a recombinant fusion protein composed of interleukin 2 (IL2) and a CD20 targeting monoclonal antibody. Pre-clinical studies have shown it maintains the activities of both antibody and cytokine components but is also involved in tumor targeting, engagement of the immune system and induction of an anti-cancer vaccine effect. In a SCID mouse model,DI-Leu16-IL2 is more effective than the individual components (IL-2 and CD20) given either alone or in combination (Gillies SD; 2005). DI-Leu16-IL2 administered intravenously to 8 relapsed/refractory Non-Hodgkin's Lymphoma (NHL) patients at a maximum dose of dose of 0.5 mg/m2 resulted in 1 complete, 1 possible partial response and 4 patients with stable disease (Nakamura R; 2013).
In this multicenter open label, dose escalation trial the safety, efficacy and tolerability of subcutaneously (SC) administered DI-Leu16-IL2 was evaluated as well as the maximum tolerated dose (MTD) and optimal biological dose in patients with relapsed or refractory B cell CD20 positive lymphoma (NCT01874288). DI-Leu16-IL2 was administered on three consecutive days every 21 days up to 6 cycles. Peripheral B cell depletion was achieved with low-dose rituximab (50mg/m2) on day 1 if needed to keep rituximab levels >10µg/mL. The starting dose was 0.5 mg/m2 and followed a modified accelerated titration until dose limited toxicity (DLT) occurred. To be evaluable for response patients had to receive at least 2 cycles of DI-Leu16-IL2 and were then evaluated by PET/CT imaging.
To date, 13 patients in 3 cohorts have been enrolled. The median age is 63 years (range, 48-83 years). Ten patients had diffuse large B cell lymphoma, 2 follicular and 1 marginal zone NHL. All were previously treated with rituximab-containing chemotherapy - median of 3 (range 1-6) prior regimens, including radiation therapy (n=5) and autologous transplantation (n=3). All patients had relapsed or refractory disease with biopsy-confirmed tumor cells expressing CD20.
DI-Leu16-IL2 dose levels were 0.5 mg/m2 (n=3), 1 mg/m2 (n=3), 2 mg/m2 (n=7). DI-Leu16-IL2 was detectable in the serum at the lowest dose level. Median number of cycles were 4 (range 1-11). No DLTs have been observed. The most common drug-related adverse events (AEs) were grade 1-2 transient skin reactions with erythema, painless induration of injection site, pruritus, edema and mild constitutional symptoms (grade 1-2 chills, low-grade fever, fatigue, low appetite) suggesting an immune stimulatory response. Lymphocyte margination occurred with nadir of 0.3x103 /mL for median 2.7 days (range 1-5). Two grade 3 non-DLT toxicities (diarrhea and QTc prolongation with pre-existing RBBB) resulted in DI-Leu16-IL2 dose reduction. Transiently prolonged QTc (grade 1-2) occurred in 3 additional patients. Routine laboratory monitoring revealed grade 1-2 transient eosinophilia, anemia and thrombocytopenia in most subjects, grade 1-2 neutropenia (n=3) without neutropenic fever, grade 1 elevation of alkaline phosphatase or bilirubin. All AEs resolved completely within one week.
Twelve patients are evaluable for response. After 2 cycles, tumor regression or stabilization was noted in 10 of 12 patients with mean tumor reduction of 30% (range 0%-80%). Six had sustained disease control after 4 cycles. One patient with small tumor bulk marginal zone lymphoma achieved a complete response by PET criteria, 3 patients had a partial response (55%, 55% and 80% tumor size reduction) and continue on therapy. Stable disease (SD) response was observed in all dose cohorts; best responses occurred at the highest dose level (2mg/m2) administered thus far. Three patients have had SD for up to 1 year.
In conclusion, we have observed promising clinical efficacy of the novel immunocytokine DI-Leu16-IL2 in relapsed/refractory B cell NHL. SC administration has permitted higher doses than could be achieved with IV treatment and the MTD has yet to be reached. DI-Leu16-IL2 is biologically active in doses up to 2mg/m2. Repetitive SC dosing elicits clinical immune activation associated with clinical activity. Further dose escalation of DI-Leu16-IL2 is in progress.
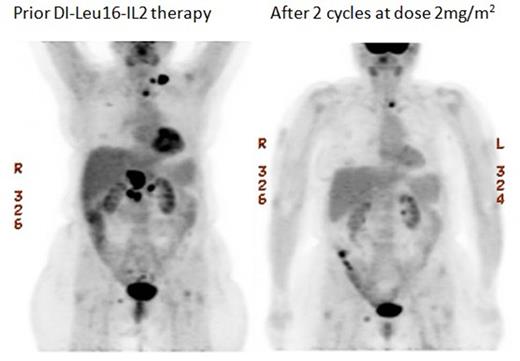
Prior DI-Leu16-IL2 therapy After 2 cycles at dose 2mg/m2
65 year old female with diffuse large B cell lymphoma treated at dose level 2mg/m2 received 2 cycles of DI-Leu16-IL2. Treatment resulted in partial regression of multiple lymph node sites.
65 year old female with diffuse large B cell lymphoma treated at dose level 2mg/m2 received 2 cycles of DI-Leu16-IL2. Treatment resulted in partial regression of multiple lymph node sites.
Bachanova:Seattle Genetics Inc.: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.