Abstract
Somatic reprogramming captures the mutations present in individual cells and can yield induced pluripotent stem (iPS) cells that can be used to study these mutations in their native genetic context. iPS cells have been made using a variety of primary tissues and established cell lines, but to date there have been few examples of somatic reprogramming using primary cancer samples. Some studies have reported iPS cell generation using samples from patients with myeloproliferative neoplasms (Ye Z Blood 2009, Hosoi M Exp. Hematol. 2014), and another study successfully reprogrammed primary bone marrow cells from patients with myelodysplastic syndromes (MDS) (Kotini AG Nat. Biotech. 2015). However, it is not yet clear whether fully transformed human myeloid leukemia cells can be reprogrammed to an undifferentiated state.
Here we describe the results of reprogramming experiments and subsequent genetic characterization of iPS clones produced from primary bone marrow and peripheral blood samples from adult human de novo AML patients. Our reprogramming approach involved in vitro culture of primary cells on Hs27 stroma with hematopoietic cytokines for 3-7 days, followed by transfer of 250,000 cells to stroma-free conditions for transduction with nonintegrating Sendai viruses expressing cMyc, OCT3/4, KLF4, and SOX2. Cells were then returned to AML culture conditions with stroma for 2-4 days before plating on mouse embryonic fibroblasts (MEF) in human embryonic stem (ES) cell media for 2-6 weeks. Individual clusters of cells with undifferentiated iPS cell colony morphology were then picked and expanded on either MEFs or feeder-free conditions. We performed 21 transductions using 8 peripheral blood and 13 bone marrow samples from 16 AML patients (i.e., multiple samples were attempted for some AMLs), which yielded 65 iPS clones from 9 of the 16 AML patients (56%) that were successfully expanded for genomic analysis. The remaining AMLs either produced no colonies (N=5), or clones that failed to expand after transferring from the original plate (N=2). Initial analysis of representative iPS clones (N=4) via flow cytometry demonstrated expression of the pluripotency markers SSEA-4 and TRA-1-60. Additional experiments to assess the pluripotency of these iPS lines are currently underway, including analysis of all clones via flow cytometry, RNA-sequencing, and teratoma formation assays.
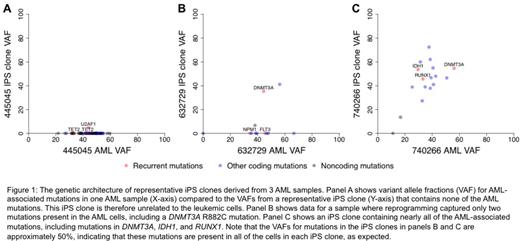
To determine the relationship between each iPS clone and the original AML samples used for reprogramming, we performed targeted sequencing for all somatic mutations identified from either whole-genome or exome sequencing. Analysis of each iPS clone for multiple patient-specific AML mutations (range 12-683) demonstrated that the reprogrammed cells were derived from 1 of 3 distinct cell types, depending on the sample. The most common type (N=1, 1, 3, 10, and 12 clones from 5 AMLs) possessed virtually no AML mutations (Figure 1A), suggesting that reprogramming occurred in a cell population that was unrelated to the tumor. Another 24 clones from 2 AML samples (N=1 and N=23) contained a subset of the AML-associated mutations (Figure 1B), but lacked common AML mutations that are generally cooperating 'hits', such as NPM1, and FLT3; for these samples, reprogramming probably occurred in a cell that was ancestral to the AML founding clone (i.e., a pre-leukemic cell). The final group of 14 clones from 2 AMLs (N=7 for both samples) contained the majority of AML-associated mutations in those samples, including canonical mutations in IDH1 and IDH2, and mutations in DNMT3A and RUNX1 (Figure 1C), implying that reprogramming occurred in the most prevalent AML subclone in the sample. Remarkably, for AML samples that yielded >1 iPS clone (N=6), all the iPS clones had the same set of mutations, suggesting that some of the cells in the sample were more "fit" for reprogramming than others.
In conclusion, we have generated iPS cell lines from 9 primary AML samples, several of which contain canonical AML mutations. In this study, the majority of the reprogramming events took place in rare cells from clones that were not the most abundant cells in the sample. However, in one case, all iPS clones were derived from the most prevalent AML subclone in the sample. Future study of these iPS cell lines will provide insights into epigenetic dysregulation in cancer, and of the functional consequences of the mutational combinations that were "captured" via reprogramming.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.