Abstract
Background: Beta thalassemia major patients (pts) are at an increased risk of heart failure, due to the deposition of iron in the heart causing myocardial siderosis. Intensive long-term iron chelation therapy (ICT) is required to obtain a normal myocardial T2* (mT2* >20 ms). Previously published studies suggested that cardiac iron removal lags changes in liver iron, and liver iron concentration (LIC) may affect the rate of removal of cardiac iron (Porter et al, ASH 2013). The objective of these analyses was to evaluate the association of the severity of LIC levels with the change in mT2* responses in pts with myocardial siderosis when treated with deferasirox (DFX) and deferoxamine (DFO) for up to 24 months (mo) in the CORDELIA study. Due to the very low pt numbers in the DFO arm, the results for these pts are not presented here.
Methods: The study design, inclusion, and exclusion criteria have been reported previously (Pennell et al, Am J Hematol. 2015). Pts were categorized into LIC <7, 7 to <15 and ≥15 mg Fe/g dry weight (here after mg/g) both at baseline (BL) and specific visits, to assess the relation of absolute LIC and changes in LIC overtime, with mT2* and cardiac iron concentration (CIC), respectively. During the study, mT2* (ms), and LIC (mg/g) were measured every 6 mo at the same time point. CIC (mg/g) was analyzed as a post hoc parameter derived from mT2*. The change in mT2* was assessed as geometric mean (Gmean)±coefficient of variation (CV), ratio of the Gmean at specific time points divided by that at BL (Gmean at specific time point/Gmean BL) and both CIC and LIC as mean±SD, unless otherwise specified.
Results: Of 197 pts, 160 (81.2%) completed 12 mo of treatment and 146 (74.1%) entered into the extension study whereas 103 pts continued on initially assigned treatment. Pts completing 24 mo of treatment included 65 (87.8%) of 74 pts (mean age 20.1±6.9 years, 59.5% male) on DFX and the results for these pts are presented as follows. Average actual doses (mg/kg/d) were 26.7±8.9, 31.5±7.4, 38.0±2.9 for LIC <7, 7 to <15, ≥15, respectively, during the extension study.
The LIC levels for pts categorized by LIC <7, 7 to <15 and ≥15 improved from BL to Mo 24 as follows: 72% decrease (mean absolute change, -15.1±14.1), 66% decrease (-26.6±13.0), and 19% decrease (-10.2±15.7), respectively.
For pts with BL LIC <7, 7 to <15, ≥15, mT2* improved from BL to Mo 24 as follows: 43% increase (14.0±18.1 to 21.6±31.1; mean abs change, 7.8±4.0), 50% increase (12.3±34.4 to 19.1±46.4; 8.0±6.0), and 30% increase (11.1±30.8 to 14.5±40.8; 4.1±5.0). The CIC values improved from BL to Mo 24 by 38% (1.8±0.4 to 1.1±0.5), 40% (2.3±0.9 to 1.4±0.7), and 23% (2.6±1.0 to 1.9±1.0), respectively.
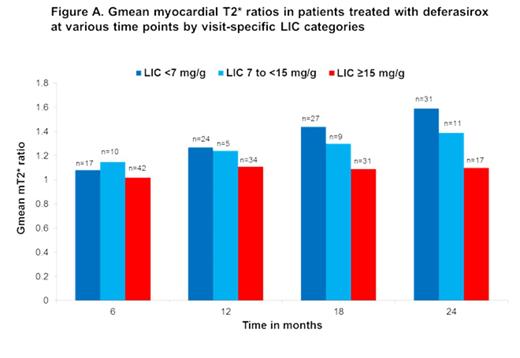
The mT2* responses for pts categorized according to visit specific LIC levels (LIC <7, 7 to <15, ≥15) from BL to Mo 12 were 22% increase (mean abs change, 3.7±4.3) in LIC <7, 21% increase (2.7±2.0) in LIC 7 to <15, and 7% increase (1.5±3.2) in LIC ≥15. From BL to Mo 24, mT2* increased by 51% (mean abs change, 7.8±5.3), 35% (4.1±2.5), and 11% (2.0±4.4), respectively. The CIC levels improved from BL to Mo 24 by 40% (mean abs change, -1.0±0.8) in LIC <7, 31% (-1.0±0.6) in LIC 7 to <15, and 6% (-0.1±0.8) in LIC ≥15. The change in mT2* (Gmean ratio) at Mo 6, 12, 18 and 24 are shown in the Figure A. The mT2* response was higher in pts who achieved a lower LIC category (LIC <7) at respective time points and this change in mT2* was more apparent at 18 and 24 mo of treatment with DFX.
Discussion: Overall, DFX treatment resulted in a substantial decrease in LIC and improved mT2*. These results suggest a greater difference in mT2* improvement and CIC reduction in pts who achieved lower LIC during treatment with DFX. This divergence was progressive with time, being maximal at Mo 24. Thus, a therapeutic response in LIC with DFX may be associated with a greater likelihood of improving mT2*. Pts with high LIC ≥15 may require an effective long-term treatment with higher doses of ICT to have an improvement in mT2*, suggesting that cardiac iron removal is likely to be slow in heavily iron overloaded pts. These results are consistent with the previous report which showed a significant decrease in LIC and increased mT2* responses at Mo 36 in pts who attained lower end-of-year LIC levels when treated with DFX (Porter et al, ASH 2013) and highlight the potential value of monitoring the liver and cardiac responses during ICT. To further understand the kinetics between liver and cardiac iron removal, prospective investigation is warranted.
Pennell:Novartis: Consultancy, Research Funding; Apotex: Consultancy, Research Funding. Porter:Celgene: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Shire: Consultancy, Honoraria. Piga:Acceleron: Research Funding; Cerus: Research Funding; Apopharma: Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding; Celgene Corporation: Honoraria. Han:Novartis: Employment. Vorog:Novartis: Employment. Aydinok:Cerus: Research Funding; Sideris: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract