Abstract
Introduction: Sickle cell anemia (SCA) is a severe monogenic hereditary disorder characterized by chronic hemolytic anemia and the occurrence of frequent painful vaso-occlusive crises (VOC). SCA classical physiological scheme involves hemoglobin S polymerization under hypoxic conditions, which triggers red blood cells (RBCs) sickling. Recent studies demonstrated that the degree of hemorheological alterations, such as blood hyper-viscosity, determines the risk for VOC. Moreover, sickle RBCs abnormally adhere to the vascular endothelium, triggering microvascular occlusions.
Despite extensive research very few drugs are available to efficiently treat VOCs or VOC-like events. A first clinical trial was performed to test the efficacy of poloxamer 188 (P188) in a large sickle cell cohort of adults and children (Orringer et al., 2001). This study demonstrated a significant reduction of pain duration in the children treated with P188. Recently, a new phase III multi-center trial has been started to test the efficacy of this drug during acute VOC in children (Humphries et al., 2015).
We conducted in vitro experiments using the commercial formulation named Kolliphor P188 (Sigma-Aldrich) to test the effects of this drug on RBCs biophysical properties of SCA patients.
Methods: To measure deformability and mechanical properties of RBCs, we used ektacytometry and microfluidic device mimicking the diameters of the micro vessels. RBCs adhesion assays were performed on HMEC-1 (Human Microvascular Endothelial Cell line) using dynamic flow adhesion platform. RBCs from healthy (AA) and SCA individuals were used for the different experiments.
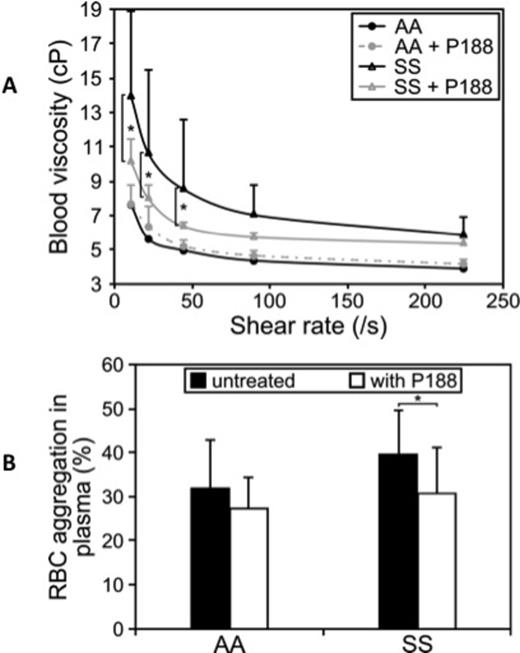
Results: While P188 did not significantly affect blood viscosity in AA, P188 treatment decreased blood viscosity at the lowest shear rates in SCA (Fig 1A). When measured in plasma, RBC aggregation decreased with P188 in SCA patients but not in AA (Fig 1B).
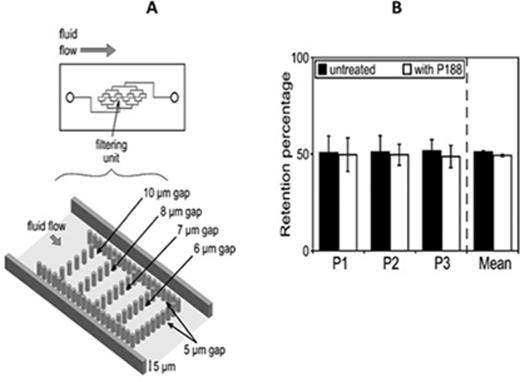
RBC deformability assessed by both ektacytometry (not shown) and microfluidic device (Fig 2) was not affected by P188. This is in agreement with the mode of action of P188 suggesting that it binds to hydrophobic surfaces and lowers surface tension without any changes in the organization of the cytoskeleton.
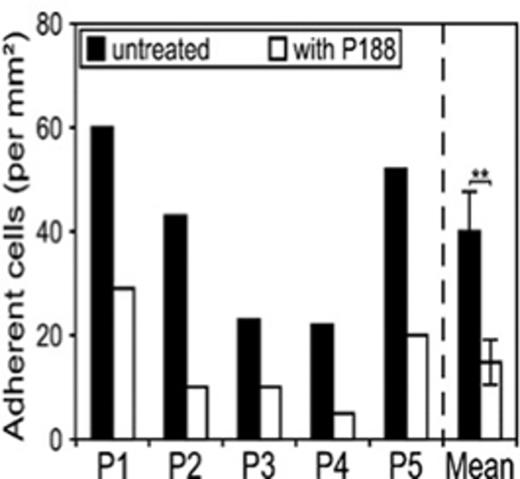
We examined the effect of P188 treatment on SCA-RBCs adhesion to the endothelial HMEC-1 cell line. We observed a mean adhesion of 40 cells/ mm2 for the untreated SCA-RBCs versus 20 cells/mm2 in the case of P188 treated RBCs, i.e. a 50% decrease upon P188 treatment (Fig 3). As for RBC aggregation, our findings suggest that the binding of P188 to SCA-RBCs membranes prevent the interaction with endothelial cells. This is of particular importance in the context of SCA since increased RBC adhesiveness has been demonstrated to trigger VOC.
Conclusion: In parallel of the phase III clinical trial studying the profit of P188 for sickle cell patients during VOCs, our results bring clarifications regarding its mode of action on RBCs. We show that P188 directly reduces blood viscosity, RBC aggregation and adhesiveness to endothelial cells, making this drug as a potential beneficial therapy in SCA.
References: Orringer, E.P., et al; (2001). JAMA, 286, 2099-2106.
Humphries, J.D., et al; (2015). Trends Cell Biol, 25, 388-397.
P188 treatment decreases blood viscosity (A) and RBC aggregation (B) in SS patients but not in AA controls.
P188 treatment decreases blood viscosity (A) and RBC aggregation (B) in SS patients but not in AA controls.
P188 treatment does not change SCA-RBC deformability. (A) Design of the microfluidic chip containing eight filtering units organized in parallel. Each filtering unit has a height of 5 µm and pillars are organized to allow an escape route of 20 µm around the unit to avoid occlusion. The surrounding pillars line has 5 µm slits. (B) Retention percentage of untreated and P188-treated SCA-RBC in 5 µm slits. Histograms represent mean of 5 µm slits from the 8 filtering units in one chip expressed in percent of total trapped RBCs for three patients.
P188 treatment does not change SCA-RBC deformability. (A) Design of the microfluidic chip containing eight filtering units organized in parallel. Each filtering unit has a height of 5 µm and pillars are organized to allow an escape route of 20 µm around the unit to avoid occlusion. The surrounding pillars line has 5 µm slits. (B) Retention percentage of untreated and P188-treated SCA-RBC in 5 µm slits. Histograms represent mean of 5 µm slits from the 8 filtering units in one chip expressed in percent of total trapped RBCs for three patients.
Graph representing adherent cells per mm2 at a flow rate of 1 dyne/mm2. The mean of the 5 patients is expressed as the average number of adherent cells/mm2 ± SD. Paired t test, P < 0.05.
Graph representing adherent cells per mm2 at a flow rate of 1 dyne/mm2. The mean of the 5 patients is expressed as the average number of adherent cells/mm2 ± SD. Paired t test, P < 0.05.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.