Introduction
Thrombophilia testing in unprovoked venous thromboembolism patients (VTE) is controversial. Common thrombophilias such as Factor V Leiden or prothrombin gene variant appear to not importantly increase the risk of VTE recurrence, and thus are not considered in anticoagulation management decisions. However, patients with potent thrombophilias such as antiphospholipid antibodies (APLA), antithrombin deficiency, protein C and S deficiency, and homozygous genetic thrombophilias or combined defects are at higher risk of recurrence and it is recommended that they receive long-term anticoagulation. If the proportion of patients with "potent" thrombophilia is high then thrombophilia testing should be conducted. We sought to determine the proportion of unprovoked VTE patients with "potent" thrombophilia.
Methods
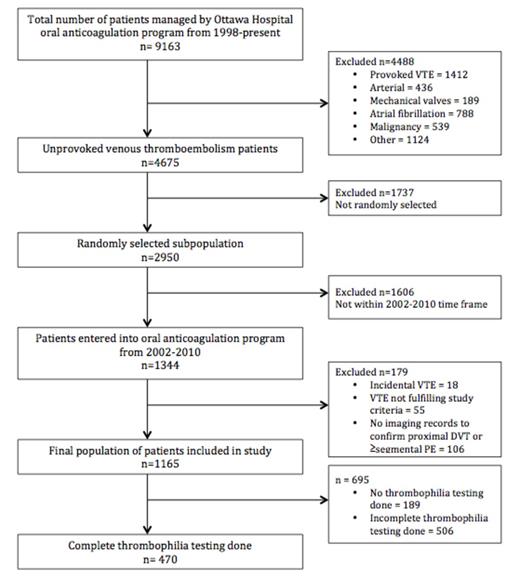
All patients with managed in our oral anticoagulation management system in the period from 1998 to 2015 were potentially eligible for the study. Inclusion criteria were: 1) symptomatic, objectively confirmed VTE unprovoked proximal deep vein thrombosis or pulmonary embolism. Exclusion criteria were: 1) cancer or myeloproliferative disease at the time of VTE diagnosis; 2) no cast, surgery, trauma or immobilization (>3 days in bed 90% of waking hours) in the 90 days prior to diagnosis. We selected unprovoked VTE patients diagnosed between 2002 and 2010, as thrombophilia testing was relatively universal and available in our electronic system in that time frame (N=1344). We then selected a convenience sample of N=1165. The primary outcome measure was the proportion of patients with "potent" thrombophilia (potent= homozygous Factor V Leiden, homozygous Prothrombin gene variant, APLA, protein C, protein S or anti-thrombin deficiency or combined deficiencies).
Results
In 1165 patients with unprovoked VTE, complete screening was done in 470 patients (40.34%) and 976 (83.78%) had at least one thrombophilia test. Complete thrombophilia testing was defined as a screen including testing for factor V Leiden, prothrombin gene defect, APLA, anti-thrombin deficiency, protein C, and protein S. Potent thrombophilias were demonstrated in 103/1165 patients (8.84%; 95% CI, 7.34 to 10.61) (Table 2) in the total study population, and 103/976 (10.55%; 95% CI, 9.62-14.47) in patients with at least one thrombophilia test.
Conclusion
The proportion of unprovoked VTE patients with "potent" thrombophilia is high. Given a high proportion of "potent' thrombophilia patients who likely benefit from indefinite anticoagulation, complete thrombophilia testing appears warranted in patients with unprovoked VTE in whom anticoagulants maybe discontinued. Thrombophilia testing is warranted for a selected group of patients to detect high-risk thrombophilias that could impact anticoagulation management.
| Thrombophilia screening . | . |
|---|---|
| Complete screening | 470 (40.3%) |
| No screening | 189 (16.2%) |
| At least one thrombophilia test | 976 (83.8%) |
| Thrombophilia screening . | . |
|---|---|
| Complete screening | 470 (40.3%) |
| No screening | 189 (16.2%) |
| At least one thrombophilia test | 976 (83.8%) |
| Thrombophilia . | All patients (n=1165) . | Tested for individual thrombophilia . | ||
|---|---|---|---|---|
| % . | 95% CI . | % . | 95% CI . | |
| FVL Heterozygous | 162/1165 (13.9%) | 12.0-16.0% | 162/883 (18.4%) | 15.9-21.0% |
| FVL Homozygous | 4/1165 (0.3%) | 0.1-0.9% | 4/883 (0.5%) | 0.2-1.2% |
| Prothrombin Heterozygous | 63/1165 (5.4%) | 4.3-6.9% | 63/831 (7.6%) | 6.0-9.6% |
| Prothrombin Homozygous | 1/1165 (0.0%) | 0.0-0.5% | 1/831 (0.1%) | 0.0-0.7% |
| Antithrombin deficiency | 10/1165 (0.9%) | 0.5-1.6% | 10/815 (1.2%) | 0.7-2.2% |
| Protein C deficiency | 18/1165 (1.6%) | 1.0-2.4% | 18/639 (2.8%) | 1.8-4.4% |
| Protein S deficiency | 13/1165 (1.1%) | 0.7-1.9% | 13/635 (2.1%) | 1.2-3.5% |
| Lupus anticoagulant | 24/1165 (2.1%) | 1.4-3.1% | 24/849 (2.8%) | 1.9-4.2% |
| Anticardiolipin IgM | 16/1165 (1.4%) | 0.9-2.2% | 16/886 (1.8%) | 1.1-2.9% |
| Anticardiolipin IgG | 20/1165 (1.7%) | 1.1-2.6% | 20/885 (2.2%) | 1.5-3.5% |
| β-2 microglobulin IgM | 10/1165 (0.9%) | 0.5-1.6% | 10/333 (3.0%) | 1.6-5.4% |
| β-2 microglobulin IgG | 8/1165 (0.7%) | 0.4-1.4% | 8/333 (2.4%) | 1.2-4.7% |
| Homocysteine | 50/1165 (5.7%) | 4.3-7.4% | 50/668 (7.5%) | 5.7-9.7% |
| Factor VIII elevated | 11/1165 (0.9%) | 0.5-1.7% | 11/646 (1.7%) | 1.0-3.0% |
| At least one or more of the above | 331/1165 (28.4%) | 25.9-31.1% | 331/976 (33.9%) | 31.0-36.9% |
| Potent thrombophilia | 103/1165 (8.8%) | 7.34-10.6% | 103/976 (10.6%) | 9.6-14.5% |
| Thrombophilia . | All patients (n=1165) . | Tested for individual thrombophilia . | ||
|---|---|---|---|---|
| % . | 95% CI . | % . | 95% CI . | |
| FVL Heterozygous | 162/1165 (13.9%) | 12.0-16.0% | 162/883 (18.4%) | 15.9-21.0% |
| FVL Homozygous | 4/1165 (0.3%) | 0.1-0.9% | 4/883 (0.5%) | 0.2-1.2% |
| Prothrombin Heterozygous | 63/1165 (5.4%) | 4.3-6.9% | 63/831 (7.6%) | 6.0-9.6% |
| Prothrombin Homozygous | 1/1165 (0.0%) | 0.0-0.5% | 1/831 (0.1%) | 0.0-0.7% |
| Antithrombin deficiency | 10/1165 (0.9%) | 0.5-1.6% | 10/815 (1.2%) | 0.7-2.2% |
| Protein C deficiency | 18/1165 (1.6%) | 1.0-2.4% | 18/639 (2.8%) | 1.8-4.4% |
| Protein S deficiency | 13/1165 (1.1%) | 0.7-1.9% | 13/635 (2.1%) | 1.2-3.5% |
| Lupus anticoagulant | 24/1165 (2.1%) | 1.4-3.1% | 24/849 (2.8%) | 1.9-4.2% |
| Anticardiolipin IgM | 16/1165 (1.4%) | 0.9-2.2% | 16/886 (1.8%) | 1.1-2.9% |
| Anticardiolipin IgG | 20/1165 (1.7%) | 1.1-2.6% | 20/885 (2.2%) | 1.5-3.5% |
| β-2 microglobulin IgM | 10/1165 (0.9%) | 0.5-1.6% | 10/333 (3.0%) | 1.6-5.4% |
| β-2 microglobulin IgG | 8/1165 (0.7%) | 0.4-1.4% | 8/333 (2.4%) | 1.2-4.7% |
| Homocysteine | 50/1165 (5.7%) | 4.3-7.4% | 50/668 (7.5%) | 5.7-9.7% |
| Factor VIII elevated | 11/1165 (0.9%) | 0.5-1.7% | 11/646 (1.7%) | 1.0-3.0% |
| At least one or more of the above | 331/1165 (28.4%) | 25.9-31.1% | 331/976 (33.9%) | 31.0-36.9% |
| Potent thrombophilia | 103/1165 (8.8%) | 7.34-10.6% | 103/976 (10.6%) | 9.6-14.5% |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.