Abstract
Introduction
Improving therapy for rel/ref AML remains a challenge. Decitabine, a DNA methyl-transferase inhibitor, initially showed promise in AML as a 5-day, first-line induction regimen and more recently as a 10-day regimen in older and unfit patients (1). However, little is known about the activity of decitabine in the rel/ref patient population despite increased use. Therefore, we sought to analyze the outcomes of these pts treated at our institution.
Methods
To obtain data regarding decitabine efficacy in rel/ref AML, we performed a retrospective analysis of outcomes following decitabine treatment in 34 adult pts treated at The University of Chicago from January 2009 to June 2014. Permission to access patient charts was granted by the medical centerÕs Institutional Review Board. AML was defined by WHO criteria, genetic risk grouping and complete remission (CR) was according to ELN classification; PR was defined as >50% decrease in bone marrow blasts and normalization of blood counts. Rel/ref AML was defined as either having had a prior CR with recurrence of disease or having received a prior induction regimen (1-2 cycles) without CR.
Results
Median pt age was 62 yrs (range, 18-81) and 60% were male. Median Charlson comorbidity index (CCI) was 5 (range, 0-8); 29% had ECOG performance status 0-1 and 71% had >2. 21 pts (62%) had de novo AML (7 with myelodysplasia-related changes), 3 (9%) had therapy-related myeloid neoplasm (t-MN), and 10 (29%) had secondary AML after myelodysplastic syndrome. 6% were in the ELN favorable genetic group, 3% intermediate-I, 18% intermediate-II, and 67% adverse; 2 cases were unevaluable. The median number of prior treatment regimens was three. 9% had received prior azacitidine, 85% had received prior HiDAC, and 38% had a prior allogeneic stem cell transplant (SCT).
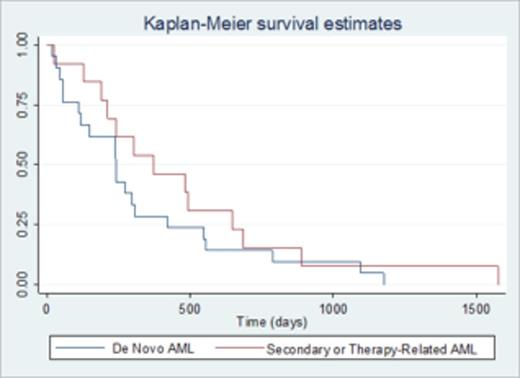
34 pts received a total of 71 cycles of decitabine, 20 mg/m2 daily, in 5 or 10-day cycles every 28 days. All patients received 10-day courses, 91% had an initial 10-day course, and 74% had only 10-day courses. The median number of cycles per pt was 2; 59% received >1 cycle. 7 (21%) achieved CR and 4 (12%) had a partial response (PR), for an overall response rate (OR) of 33%. Responses occurred in 24% of pts with de novo AML, 66% with t-MN, and 50% with secondary AML. Intermediate and adverse group pts had OR of 14% and 39%, respectively. All pts achieving CR did so after 1 cycle; PR required a median of 3 cycles. Pts who achieved CR or PR had a significantly lower pretreatment WBC count (median, 9.5 vs 49.5 x 103/µL in non-responders; p=0.015) and blast percentage (44 vs 59.4; p=0.035) than those who did not. Pts with secondary AML or t-MN had a higher probability of OR compared to those with de novo AML (54 vs 23%; p=0.042). Median overall survival (OS) of all pts was 256 days; prior SCT was associated with reduced OS (p=0.017). When comparing de novo to secondary AML & t-MN, 1-year OS was not significantly different (Figure 1). Responders had a significantly longer OS (median, 622 days vs 278 days for non-responders; p=0.012). Age, race, CCI, ECOG PS, genetic risk group, prior HiDAC, dysplasia, azacitidine, and number of prior treatments did not impact OR or OS. 16 (47%) pts proceeded to SCT. During treatment, 70% had a grade 3-4 non-hematologic toxicity (based on NCI CTACE v4.0); the most common was fatigue. The median number of hospitalizations for complications per patient was 2 (range, 0-7). Causes of hospitalization were febrile neutropenia (40%), infection (22%), cytopenias (18%), rash (6%), acute kidney injury (6%), and 8% were for other causes.
Conclusion
Decitabine treatment of 34 adults with rel/ref AML resulted in an OR of 33% (21% CR) and allowed nearly one-half of these pts to proceed to SCT. All pts achieving CR did so after 1 cycle. Responding pts had improved OS over those without response (p=0.012). Interestingly, secondary AML or t-MN were 7.8 times more likely to achieve a response compared to de novo AML (p=0.046); lower WBC count and marrow blast percentage also correlated with higher OR. Further delineation of molecular subsets associated with response to decitabine should be evaluated in a larger prospective trial in this high-risk AML population.
Citation
1. Blum KA, et al. Phase I trial of low dose decitabine targeting DNA hypermethylation in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: dose-limiting myelosuppression without evidence of DNA hypomethylation. Br J of Haem. Jul 2010;150(2):189-195.
Off Label Use: Decitabine is indicated for treatment of MDS but is often used to treat newly diagnosed or relapsed/refractory AML. In this study we analyzed results of patients with AML who were treated with decitabine in the relapsed/refractory setting.. Thirman:AbbVie: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Gilead: Research Funding; Merck: Research Funding; AbbVie: Research Funding; Gilead: Research Funding; Merck: Research Funding. Odenike:Sunesis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Liu:Astra Zeneca/Medimmune: Consultancy; Pfizer: Consultancy; Astra Zeneca/Medimmune: Consultancy; Pfizer: Consultancy. Stock:Gilead: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.