Abstract
Background: Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of non-Hodgkin lymphomas associated with poor prognosis and repeated recurrence for most subtypes. Currently, anthracycline-based therapies such as cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or CHOP-like therapies are recommended as the first-line treatment for PTCL, but the prognosis remains poor with most patients relapsing within 5 years. Thus, improved treatment strategies are still needed.
Belinostat is a potent, pan-histone deacetylase inhibitor that was recently approved in the United States for the treatment of patients with relapsed or refractory PTCL (R/R PTCL). Approval was based on results from the pivotal Phase 2 BELIEF study (O'Connor et al, JCO, 2015) of belinostat in R/R PTCL, which demonstrated durable clinical benefit (objective response rate [ORR] 25.8%) and tolerability. Since belinostat (Bel) and each of the components of the CHOP regimen target different aspects of the cell cycle with different mechanisms of action, there is potential for a synergistic effect of a Bel-CHOP combination treatment regimen for patients with PTCL.
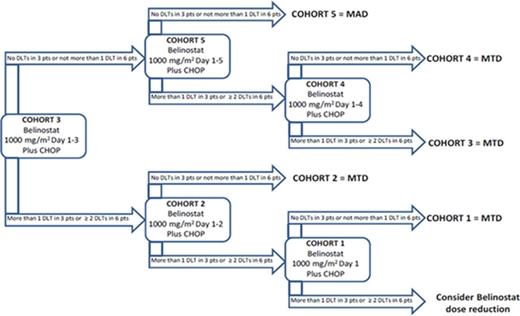
Methods: Patients with PTCL received CHOP in association with 1000 mg/m2 of belinostat on various schedules, repeated every 21-days for up to 6 cycles. The cohort schema followed a traditional "3+3" dose escalation design. The objective of Part A of the study was to determine the Maximum Tolerated Dose (MTD) of the Bel-CHOP combination. Once the MTD was determined, at least 10 more patients were to be treated in the Expansion Phase (Part B). Belinostat was to be administered as a 1000 mg/m2 IV infusion once daily for up to 5 days, depending on the assigned cohort (Fig 1). The starting cohort was Cohort 3 (CHOP + 1000 mg/m2 of daily belinostat on Days 1-3).
Patients received primary prophylaxis with growth factor (G-CSF) support. Dose-limiting toxicities (DLT) were considered during the 1st cycle and included: non-hematological toxicity Grades 3-4, platelet count < 25 X 109/L at any time or ANC < 0.5 X 109/L lasting more than 7 days despite G-CSF administration. The primary endpoint of the study was the determination of the MTD of the Bel-CHOP combination. Secondary endpoints included safety, tolerability and ORR (complete response [CR] + partial response [PR]) and pharmacokinetics.
Results: A total of 23 patients were enrolled in the study, 11 of which were treated in Part A. One patient in Part A was deemed inevaluable because the patient died due to disease progression before completing Cycle 1. The MTD was determined to be 1000 mg/m2 on Days 1-5 (Cohort 5); 12 more patients were then treated at this dose level (Part B). The only DLT experienced in the study was in Cohort 3 (Grade 3 Nausea and Vomiting). At the time of this abstract, 18/23 patients (78%) have completed all 6 cycles of Bel-CHOP, with 87% completing at least 4 cycles. Ten patients (43%) had at least one serious adverse event (SAE) and 18 (78%) had at least one Grade 3 or 4 adverse event (AE). The most frequent Grade 3/4 AEs were hematological in nature: neutrophil count decreased (26%), anemia (22%), neutropenia (17%) and white blood cell count decreased (17%). The ORR for the18 patients that have completed an End of Study Visit is 89% (16/18), with the vast majority achieving a CR [72% (n=13)], and 17% (n=3) a PR. Progressive disease was reported in 2 patients.
Conclusions: These results demonstrate that the combination of belinostat with CHOP (Bel-CHOP) is well tolerated, with all components of CHOP and belinostat being given at their standard therapeutic doses. The rates of AEs were consistent with those typically reported with CHOP alone, and clinical activity was demonstrated with a response rate of 89% based on 18 evaluable patients. Thus, Bel-CHOP is a promising new regimen in PTCL that will be further tested in a Phase 3 randomized trial.
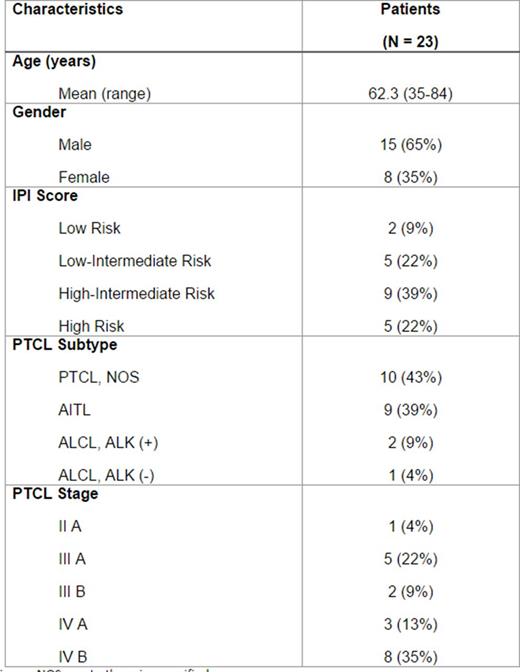
Summary of Demographic and Baseline Characteristics
AITL= angioimmunoblastic T-cell lymphoma; ALCL =anaplastic large-cell lymphoma; ALK = anaplastic lymphoma kinase; NOS = not otherwise specified
Summary of Demographic and Baseline Characteristics
AITL= angioimmunoblastic T-cell lymphoma; ALCL =anaplastic large-cell lymphoma; ALK = anaplastic lymphoma kinase; NOS = not otherwise specified
Barta:Seattle Genetics: Research Funding. Bhat:Spectrum Pharmaceuticals, Inc: Employment. Song:Spectrum Pharmaceutical, Inc: Employment. Choi:Apectrum Pharmaceuticals, Inc: Employment. Allen:Spectrum Pharmaceuticals, Inc: Employment. Foss:Spectrum Pharmaceuticals; Celgene: Seattle Genetics: Infinity; Millenium: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.