Abstract
Background: Follicular lymphoma (FL) is the most common indolent NHL with a heterogenous natural history of disease and a median survival of 8 to 12 y, albeit ranging between 1 to 20 y. Casulo et al. (2015) identified a high-risk FL cohort of patients with progression of disease (POD) at ≤24 months following initiation of immunochemotherapy with R-CHOP. Idelalisib (Zydelig) is a first-in-class, highly selective, oral inhibitor of PI3Kd which is indicated for relapsed FL or SLL following receipt of at least two lines of systemic chemotherapy. Retrospective subgroup analysis of the idelalisib registrational trial NCT01282424 (101-09) of a cohort with early POD following immunochemotherapy was performed to assess possible activity of idelalisib in this population.
Methods: A subset of 46 patients enrolled in study 101-09 were identified as having been diagnosed with FL and having received first-line immunochemotherapy, of which 37 experienced early POD, defined as starting second-line treatment within 24 months of initial first-line treatment. For the latter group, descriptive statistics of demographic and baseline characteristics and inter-treatment intervals in months as well as Kaplan-Meier estimates of progression-free survival (PFS) and overall survival (OS) following initiation of immunochemotherapy and idelalisib were calculated.
Population: Demographic characteristics of these 37 patients included median (range) age at initiation of idelalisib of 64 (33-84) y and 18 (48.6%) females. Histologic grade at diagnosis included 33 (89.2%) with grades 1 or 2 as well as 4 (10.8%) with grade 3A, while 21 (56.8%) patients had a FLIPI score ≥3. The mean (s.d.) number of prior therapies was 3.4±1.4, with a range of 2 to 8, while first-line therapies included 21 (56.8%) patients who received R-CHOP-based regimens, 7 (18.9%) who received BR, and 5 (13.5%) who received R-CVP. Mean (s.d.) inter-treatment intervals included 12.5±6.1 months between first- and second-line for all patients, 9.7±9.3 months between second- and third-line for all patients, 11.9±12.0 months between third- and fourth-line for 24 (64.9%) patients, and 11.8±7.6 months between fourth- and fifth-line for 15 (40.5%) patients. Median (range) time from first-line therapy to idelalisib initiation was 30.3 (8.9-94.7) months; no patient received idelalisib as second-line therapy.
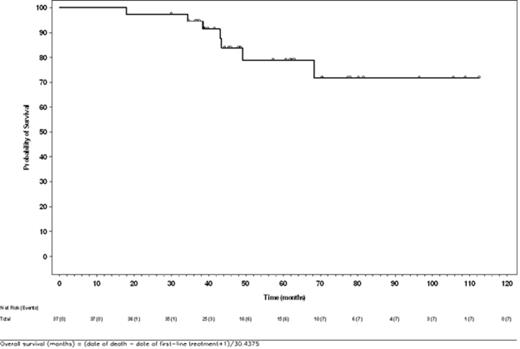
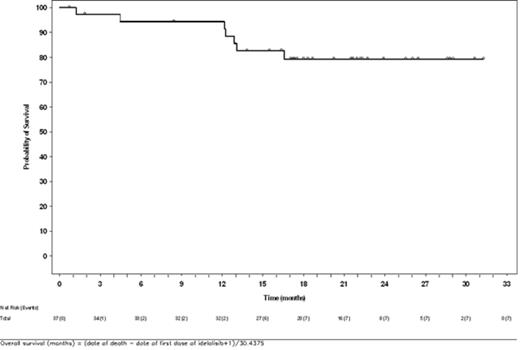
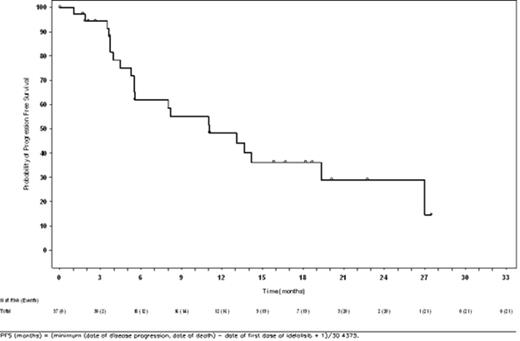
Results: Best responses included 5 (13.5%) patients with CR, 16 (43.2%) with PR, 2 (5.4%) with SD, and 1 (2.7%) with PD; median duration of response for those with CR or PR was 11.8 months (95% CI: 3.8 months, not evaluable). There were 7 (18.9%) deaths and 21 (56.8%) PFS events in this group. Estimated probabilities of survival (s.e.) and progression-free status at 2 y following initiation of idelalisib were 79%±7% and 29%±10%, respectively. Median PFS was 11.1 months (95% CI: 5.5, 19.3 months). Estimated probability of survival (s.e.) at 5 years following initiation of first-line treatment was 79%±8%. Median overall survival from both initiation of first-line immunochemotherapy as well as with idelalisib was not reached during the course of this study.
Conclusions: Idelalisib may have significant clinical activity in high-risk and doubly-refractive FL following early relapse status post first-line immunochemotherapy. Given the small size of the studied subset population which may not be representative, further characterization in additional patients is warranted to ensure the generalizability of this finding, including consideration of further investigational protocols featuring targeted therapies employed both as single agents and in combination.
Overall Survival From Initiation of First-line Treatment
Overall Survival From Initiation of First-line Treatment
Overall Survival From Initiation of Idelalisib
Progression-Free Survival From Initiation of Idelalisib
Gopal:Gilead, Spectrum, Pfizer, Janssen, Seattle Genetics: Consultancy; Spectrum, Pfizer, BioMarin, Cephalon/Teva, Emergent/Abbott. Gilead, Janssen., Merck, Milennium, Piramal, Seattle Genetics, Giogen Idec, BMS: Research Funding; Millennium, Seattle Genetics, Sanofi-Aventis: Honoraria. Kahl:Roche/Genentech: Consultancy; Seattle Genetics: Consultancy; Millennium: Consultancy; Cell Therapeutics: Consultancy; Celgene: Consultancy; Infinity: Consultancy; Pharmacyclics: Consultancy; Juno: Consultancy. Flowers:Infinity Pharmaceuticals: Research Funding; Genentech: Research Funding; Spectrum: Research Funding; Millennium/Takeda: Research Funding; Seattle Genetics: Consultancy; Spectrum: Research Funding; Pharmacyclics: Research Funding; AbbVie: Research Funding; AbbVie: Research Funding; Gilead Sciences: Research Funding; Pharmacyclics: Research Funding; Onyx Pharmaceuticals: Research Funding; Celegene: Other: Unpaid consultant, Research Funding; OptumRx: Consultancy; Gilead Sciences: Research Funding; Janssen: Research Funding; Acerta: Research Funding; Millennium/Takeda: Research Funding; Acerta: Research Funding; Infinity Pharmaceuticals: Research Funding; Janssen: Research Funding; Onyx Pharmaceuticals: Research Funding. Martin:Janssen: Consultancy, Honoraria; Acerta: Consultancy; Gilead: Consultancy; Celgene: Consultancy; Novartis: Consultancy; Bayer: Consultancy. Link:Genentech: Consultancy, Research Funding; Kite Pharma: Research Funding. Ansell:Bristol-Myers Squibb: Research Funding; Celldex: Research Funding. Ye:Gilead: Employment. Koh:Gilead: Employment. Abella:Gilead: Employment. Barr:Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Abbvie: Consultancy; Gilead: Consultancy. Salles:Calistoga Pharmaceuticals, Inc.; Celgene Corporation; Genentech, Inc.; Janssen Pharmaceutica Products, L.P.; Roche: Consultancy; Celgene Corporation; Roche and Gilead Sciences: Research Funding; Celgene Corporation; Roche: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.