Abstract
Introduction: Factor D is a highly specific serine protease that cleaves factor B as its only substrate. It is the rate-limiting step of the alternative pathway of complement (APC). Therefore, factor D is a promising therapeutic target in diseases of excess activation of the APC, such as paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). Terminal complement inhibition by eculizumab is currently the treatment of choice for PNH and aHUS. Eculizumab must be administered intravenously and indefinitely in PNH; moreover, up to 20% of PNH patients treated with eculizumab have symptomatic hemolysis due to extravascular hemolysis. Glycosylphosphatidylinositol (GPI) anchor protein deficient erythrocytes from PNH patients or erythrocytes from animals can be used to test novel complement inhibitors for PNH; however, there are no in vitro assays available to model complement-mediated attack in aHUS. Here, we demonstrate that the modified Ham test (PIGA-null reagent cell line exposed to serum from aHUS patients) is the first reliable in vitro model to test complement inhibition for aHUS. In addition, we demonstrate that small molecule factor D inhibitors specifically block the APC in PNH and aHUS.
Method: Three factor D inhibitors (ACH-3856, ACH-4100, ACH-4471) that reversibly bind factor D and are being developed for oral administration were studied. We obtained blood samples from 5 PNH and 4 aHUS patients after written informed consent. Serum and erythrocytes from PNH patients on eculizumab were collected within 60 minutes of eculizumab administration. All 3 compounds were tested in half-log dilutions ranging from 0.001 ìM to 10 ìÌ. The binding affinity to factor D was determined by surface plasmon resonance (Biacore) analysis. The inhibitory effect on factor D protease was evaluated biochemically using a natural substrate consisting of C3b and the complement factor B. The inhibition of APC-mediated hemolysis was first evaluated with rabbit erythrocytes incubated with 8% normal human serum (NHS). The inhibition of APC-mediated C3 fragment deposition was also initially evaluated with rabbit erythrocytes incubated with 20% C5-depleted NHS (to mimic the effect of eculizumab) by flow cytometry. To confirm the observation with rabbit erythrocytes, erythrocytes from PNH patients were also tested in the hemolytic assay (20% acidified NHS). Regarding aHUS, the inhibitory effect of the compounds was evaluated in the recently described modified Ham test. Briefly, PIGA-null TF-1 cells were incubated with 20% of aHUS serum for 30 minutes at 37 o C and complement-mediated cell killing was evaluated using the cell proliferation reagent (WST-1). Absorbance was measured and expressed as a ratio to the heat-inactivated control (% non-viable cells)
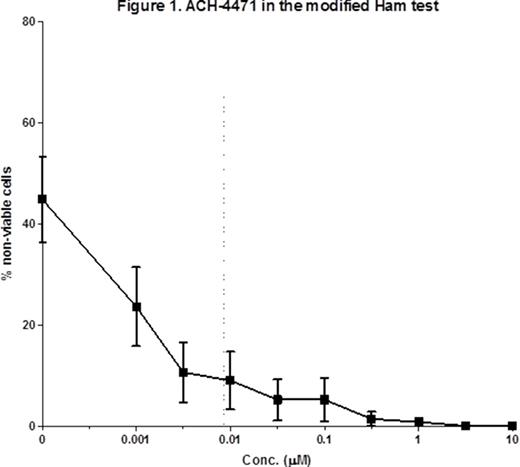
Results: All 3 small molecules showed high binding affinity to human factor D and effectively inhibited factor D proteolytic activity in a dose-dependent manner (mean IC50 9.8-20 nM). Similarly, we observed a dose-dependent inhibition of hemolysis on rabbit erythrocytes (EC50 9-17 nM). C3 fragment deposition on rabbit erythrocytes after incubation with C5-depleted serum was reduced to undetectable levels with all 3 compounds from concentrations of 0.1 ìÌ and above. Next, we studied samples from 5 PNH patients (2 treatment naïve and 3 on eculizumab) and 4 aHUS patients (1 on eculizumab, 1 in acute phase and on eculizumab and 2 in remission). When tested on the hemolytic assay with erythrocytes from PNH patients and acidified NHS, factor D inhibitors significantly reduced hemolysis in a dose-dependent manner from concentrations as low as 0.01 ìM. Lastly, cell killing was observed as previously reported in the presence of the serum from aHUS patients in the modified Ham test and addition of factor D inhibitors caused significant dose-dependent reduction in the cell killing at concentration as low as 0.01 ìÌ. Mean percentage of non-viable cells before and after addition of compound ACH-4471 is shown in Figure 1.
Conclusions: We demonstrate that factor D inhibitors, potentially the first-ever oral complement inhibitors, efficiently block hemolysis of PNH cells in vitro and mitigate the accumulation of C3 fragments. Importantly, our findings indicate that the recently described modified Ham test can be used as a preclinical model to test complement inhibitors in aHUS.
Thanassi:Achillion Pharmaceuticals: Employment. Yang:Achillion Pharmaceuticals: Employment. Huang:Achillion Pharmaceuticals: Employment. Brodsky:Alexion Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract