Abstract
Background: Monoclonal antibodies (mAbs) are an emerging therapeutic class for MM patients (pts). Elotuzumab, a mAb in late-phase clinical development, targets the SLAMF7 receptor expressed highly on MM cells. While its primary mechanism of action is through CD16-mediated ADCC, elotuzumab can also directly activate SLAMF7-expressing NK cells. Gaining a greater understanding of phenotypic and functional changes in NK cells over the course of the disease, and how these changes impact capacity for ADCC, may help identify profiles that can better select pts likely to benefit from elotuzumab or other mAb therapies.
Methods: We prospectively performed a comprehensive flow cytometry-based analysis of lymphocyte subsets, focusing on expression of NK cell activating and inhibitory receptors, activation and maturation markers, and degranulation in 30 MM pts (12 newly-diagnosed (ND), 18 relapsed/refractory (RR)) and 19 aged-matched healthy donors (HD). Over 140 immune parameters were analyzed, with differences in expression between HD and pt subsets compared by Wilcoxon rank-sum test. We analyzed correlations between expression of certain markers with each other, and with elotuzumab-induced NK cell degranulation against MM cell targets (MM1R) in a 2-hour co-culture assay. We also compared NK cell parameters in blood and bone marrow (BM) from pts with matched samples available.
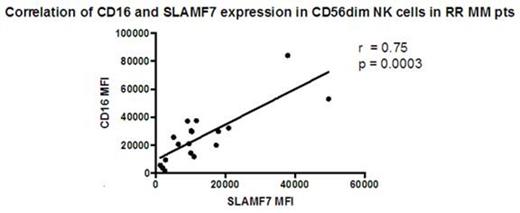
Results: Within the blood, there was no difference in relative NK cell frequency between the groups, and little difference phenotypically between HD and ND pt NK cells, except for decreased DNAM1 expression in ND. In contrast, in comparison to HD, CD56dim NK cells in RR pts were less mature with a higher CD56bright to CD56dim NK cell ratio and reduced expression of the terminal differentiation/maturation markers, CD57 and KLRG1. RR pts also showed increased expression of the activation marker CD69 on all NK cells, and their CD56dim NK cells had increased levels of the natural cytotoxicity receptors, NKp30 and NKp46 and decreased expression of activating receptors DNAM1 and NKG2D. SLAMF7 expression was also increased in RR pts, but only on the CD56bright subset. Consistent changes in NK cell expression of checkpoint/co-stimulatory molecules (eg. PD-1, Tim3, LAG3, CD137) were not seen. Despite these phenotypic changes, no significant differences between groups were noted for elotuzumab-induced ADCC against MM1R targets, as measured by CD107a degranulation by CD56dim NK cells, with significant variability noted within groups. Interestingly, the expression levels of SLAMF7 on CD56dim NK cells directly correlated with CD16 levels, particularly within RR pts (Fig.), suggesting cooperative interactions between these receptors that may be beneficial in MM patients treated with elotuzumab. In addition, degranulation toward elotuzumab-treated MM1R targets was significantly associated with surface expression levels of both SLAMF7 and CD16 on the CD56dim NK cells.
The status of NK cells was also compared between matching blood and BM samples from ND (n=7) and RR (n=8) pts. NK cell phenotype and degranulation in blood and BM were similar in ND pts, but in RR pts, expression of CD69 and SLAMF7 were higher on BM-derived NK cells, and CD56dim NK cells from BM demonstrated greater degranulation toward elotuzumab-treated MM1R targets. DNAM1 expression was reduced, but NKG2D, NKp30, and NKp46 were upregulated on various NK cell populations in BM from RR pts compared to peripheral blood.
Conclusions: Taken together, our data indicate that NK cells in RR MM pts had increased activation, reduced maturation status, and distinct changes in activating receptor expression levels that are often further enhanced in the BM microenvironment. Furthermore, CD56dim NK cells in many RR pts had parallel increased expression levels of CD16 and SLAMF7, which correlated with enhanced degranulation toward elotuzumab-treated MM target cells. The fact that these changes are seen primarily in RR pts rather than untreated ND pts implies a significant impact of disease evolution and prior therapy on the NK cell compartment, and supports further exploration of these parameters as potential biomarkers of activity of elotuzumab and other therapeutic mAbs in myeloma.
Campbell:Bristol-Meyers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cohen:Bristol-Meyers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.