Abstract
Introduction: Use of chimeric Antigen Receptor (CAR) targeting CD19 (CTL019) as a cell-based immunotherapy has been reported to have positive results with high complete response rates in hematological malignancies, including relapsed/refractory (r/r) acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphomas (NHL). In all the studies reported thus far with CTL019, the cell therapy was processed in an academic center (University of Pennsylvania). For large-scale manufacturing of CTL019 and more widespread distribution to patients and physicians, a focus on scalability to meet demands is critical. Here we report the outcomes from successful transfer of CTL019 processing technology from academia to our large-scale GMP facility.
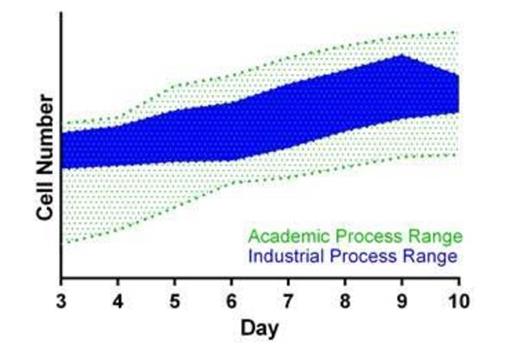
Methods & Results: The focus of effective transfer included areas of manufacturing process and analytical technology to consistently manufacture CTL019 with scale-up capabilities. Through strong collaboration of diverse technology transfer team participants from academia, GMP production, Technical development, Quality Assurance and Regulatory, we developed a step-based approach for process transfer. After gathering data from the academia process we made improvements to further enhance control and consistency of the process by implementing key Quality Systems elements (e.g., batch record, change control, process SOPs). Areas of improvement included closing of process steps through customized consumable and equipment solutions; replacing some manual processes with automation solutions and developing a new quantitation method for the expression of the CTL019 transgene. Initial production and release testing results and experience were gained by utilizing healthy donor starting material for manufacturing the cell product in our large scale GMP production facility. Patient-derived autologous CTL019 for treatment of pediatric patients with r/r ALL enrolled in a US-based, multicenter, phase II clinical trial have now been processed in the industry setting. Shipping logistics of the cryopreserved products (apheresis and CTL019) have been established. The cell expansion growth curves and release criteria on the cell products obtained in this large scale manufacturing facility were similar to those obtained at the academic facility.
Conclusions: Leveraging a proven step-wise industry transfer process to capture academic experience along with extensive collaborative training and strong analytics led to this successful CAR cell therapy process transfer from academia to industry. This resulted in CTL019 cell expansion growth curves from our process that were similar to those observed from academia, which we anticipate will provide for global scalability.
Boyd:Novartis Pharmaceuticals Corporation: Employment. Levine:Novartis: Patents & Royalties, Research Funding. Jinivizian:Novartis Pharmaceuticals Corporation: Employment. Jeschke:Novartis Pharmaceuticals Corporation: Employment. Suhoski Davis:Novartis: Patents & Royalties. Zheng:Novartis: Patents & Royalties. Stark:Novartis Pharma AG: Employment. Loidolt:Novartis Pharmaceuticals Corporation: Employment. Keir:Novartis Pharmaceuticals Corporation: Employment. Wood:Novartis Pharmaceuticals Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.