Abstract
There is increasing evidence that many patients with sickle cell disease (SCD) develop chronic pain in addition to experiencing acute pain secondary to vaso-occlusive episodes (VOE). Those patients who develop chronic pain often exhibit features suggestive of neuropathic processes such as allodynia and pain "shooting" or "tingling" in character. Using electrophysiologic techniques in ex-vivo nerve-skin preparations and nocifensive behavior studies in sickle cell mouse models, we and others have shown that SCD is associated with sensitization of light touch cutaneous sensory fibers, and decreased threshold in response to 2000Hz and 250 Hz electrical sine wave stimulation. These findings are indicative of Aβ and Ad fibers sensitization and in turn compatible with neuropathic process.
We hypothesized that the same phenomena of nerve fiber sensitization in the mouse model would be observed in SCD patients. This hypothesis was tested using an experimental paradigm with sine-wave electrical stimulation delivered at three different frequencies: 2000, 250, and 5 Hz, which preferentially stimulate Aβ, Ad, and C fibers, respectively. Thermal and mechanical sensory testing was also performed. After IRB approval, SCD subjects with high (≥ than 3 ER visits or admissions for VOEs per year) or low (< 2 ER visits or admissions per year) pain frequency, and age- and sex-matched African-American healthy controls were recruited at baseline states during routine clinic visits. After informed consent (and/or assent) was obtained, patients underwent quantitative sensory testing (QST) that included heat and cold perception and tolerance (with a TSA II-Sensory Analyzer), mechanical (using a Wagner FDIX Force One) and current perception and pain tolerance thresholds (using the Neurometer).
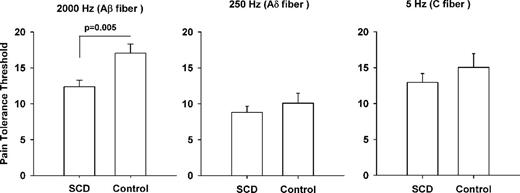
We enrolled 19 SCD subjects with high and 4 with low pain frequency (total 23 SCD participants), and 11 African-American non-SCD subjects who tolerated and completed all QSTs. As there was no difference between QST responses in patients with high and low pain frequency, these two groups were combined as the SCD group. We found that SCD subjects had significantly decreased cold tolerance threshold (p=0.034) and a trend towards lower thresholds for cold perception and heat tolerance, which is in concert with previously reported findings. Expanding upon these findings, SCD patients had a significantly reduced pain tolerance threshold in response to 2000 Hz stimulation (p=0.005), which preferentially stimulates Aβ fibers (see figure). These results suggest that adolescents with SCD have sensitization of myelinated Aβ fibers. Interrogation of the Ad and C fibers with 250 and 5 Hz stimulation, respectively, revealed no significant differences in tolerance thresholds among the groups, however SCD patients showed a trend toward lower thresholds in both frequencies (see figure). Combined, these findings support the use of sine-wave stimulation on the evaluation of pain phenotype in SCD and the notion that SCD subjects may have neuropathic processes contributing to their pain phenotype.
Demographics, thermal and mechanical sensory testing in SCD and control subjects.
| Variable . | SCD (n=23) . | Control (n=11) . |
|---|---|---|
| Age (years) | 16 (15-18.8) | 19 (14-21) |
| Male (%) | 3 (30%) | 11 (48%) |
| Cold Perception (°C) | 29.8 (28.5-30.5) | 30.4 (29.9-30.9) |
| Cold Tolerance (°C) | 8.5 (2.0-17.0) * | 0.9 (0.0-6.9) |
| Heat Perception (°C) | 34.4 (33.5-35.2) * | 33.6 (33.2-34.1) |
| Heat Tolerance (°C) | 48.9 (47.5-49.8) | 50.0 (45.3-50.1) |
| Mechanical Tolerance (lbF) | 7.7 (5.9-9.7) | 9.5 (7.7-10.8) |
| Variable . | SCD (n=23) . | Control (n=11) . |
|---|---|---|
| Age (years) | 16 (15-18.8) | 19 (14-21) |
| Male (%) | 3 (30%) | 11 (48%) |
| Cold Perception (°C) | 29.8 (28.5-30.5) | 30.4 (29.9-30.9) |
| Cold Tolerance (°C) | 8.5 (2.0-17.0) * | 0.9 (0.0-6.9) |
| Heat Perception (°C) | 34.4 (33.5-35.2) * | 33.6 (33.2-34.1) |
| Heat Tolerance (°C) | 48.9 (47.5-49.8) | 50.0 (45.3-50.1) |
| Mechanical Tolerance (lbF) | 7.7 (5.9-9.7) | 9.5 (7.7-10.8) |
*p < 0.05. Values are shown as median and interquartile range. SCD subjects reached cold tolerance and heat perception at significantly higher temperatures as compared to controls; p=0.034 and p= 0.035, respectively.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.