Abstract
Background. The prognostic significance of bone marrow (BM) fibrosis grade in pts with primary myelofibrosis (PMF) is debated. A fibrosis grade greater than 1 was associated with a 2-fold higher risk of death compared with pts with early/prefibrotic MF (grade 0) [Thiele J, Ann Hematol 2006]. Recent data suggest that more accurate prediction of survival is achieved when fibrosis grade is added to IPSS [Verner C, Blood 2008; Giannelli U, Mod Pathol 2012].
Aim. To analyze the prognostic impact of fibrosis in diagnostic BM samples of 540 WHO-2008 diagnosed PMF pts with extensive clinical and molecular information collected in 6 Italian centers belonging to AGIMM (AIRC-Gruppo Italiano Malattie Mieloproliferative).
Methods. The clinical variables assessed were those previously identified as prognostically relevant in the IPSS score. Published methods were used to screen mutations of JAK2, MPL, CALR, EZH2, ASXL1, IDH1/2 and SRSF2. European consensus scoring system was used to grade fibrosis (on a scale of MF-0 to MF-3). The prognostic value of fibrosis with regard to overall survival (OS) was estimated by Kaplan-Meier method and Cox regression.
Results. Pts' median age was 61y; median follow-up 3.7y; median OS 10.5y; 184 pts (34.1%) died. IPSS risk category: low 33.7%, Int-1 27.7%, Int-2 19.1%, High-risk 19.5%. Mutational rate: JAK2 V617F 62.6%, CALR 20.7% (type-1/1-like 77.7%, type2/2-like-2 21.4%), MPL W515 5.9%; 62 (11.5%) were triple negative (TN). 171 pts (31.7%) were High-Molecular Risk (HMR) category (Vannucchi AM, Leukemia 2013); mutation rate: EZH2 7.2%, ASXL1 22.2%, IDH1-2 2.4%, SRSF2 8.3%. According to fibrosis grading, 50 pts were MF-0 (9.3%), 180 MF-1 (33.3%), 196 MF-2 (36.3%), 114 MF-3 (21.1%).
Compared with both MF-0 and MF-1, MF-2 and MF-3 pts presented more frequently constitutional symptoms (P<.0001), larger splenomegaly (P<.0001), greater risk of developing anemia (P<.0001) or thrombocytopenia (P=.003). We found a significant association (P<.0001) between IPSS higher/Int-2 risk categories and MF-2 and -3 (20.5% and 37.8%, respectively, vs 14.8% and 6.0% for MF-0 and -1).
There was no correlation between fibrosis grade and phenotypic driver mutations; in particular, TN pts were equally distributed among MF fibrosis grades (10%, 10.6%, 14.3% and 8.8% from MF-0 to -3, respectively). Conversely, the frequency of HMR pts increased progressively according to fibrosis grade: 8 pts MF-0 (16%), 46 MF-1 (25.6%), 66 MF-2 (33.7%) and 51 MF-3 (44.7%) (P<.0001). In particular, we found a significant association between fibrosis grade and ASXL1 (12%, 15%, 23.5% and 36% from MF-0 to -3; P<.0001) and EZH2 (2%, 3.9%, 8.2%, 13.2%; P=.01) mutations. Also, pts with 2 or more HMR mutated genes were preferentially MF-2 or -3 ( 0%, 4.4% 10.2% and 10.5% from MF-0 to -3; P=.001).
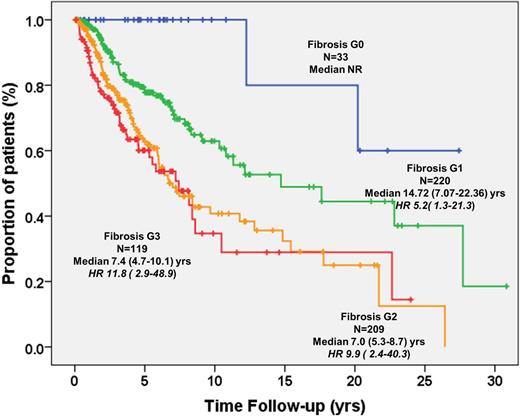
Median OS was significantly shorter in pts with MF-2 (OS 6.7y, HR 7.3, IC95% 2.7-20.0; P<.0001) and MF-3 (OS 7.2y, HR 8.7, IC95% 3.1-24.2; P<.0001) compared with MF-1 (14.7y; HR 3.9, IC95% 1.4-10.9, P=.008) and MF-0 (P<.0001) used as reference group (OS not reached) (Figure). Excluding MF-0, MF-2 and -3 maintained negative prognostic impact with HR 1.9 (1.3-2.6; P=.001) and 2.2 (1.5-3.3; P<.0001) respectively vs MF-1. The impact of fibrosis on OS was maintained when analysis was restricted to younger (≤65y) pts. In multivariate analysis using the individual IPSS variables, grade MF-2 and -3 were independently predictive of survival (HR 3.9 (1.4-10.8), and HR 4.2 (1.5-12.0), respectively, P=.008 for both). The negative impact on survival of MF-2/-3 was maintained regardless of IPSS category, HMR status, number of HMR mutated genes and driver mutations, included as covariates (Table). In low, Int-1 and Int-2, but not high-risk IPSS categories, MF-2/-3 associated with reduced survival (P<.03).
Conclusions. Overall, these results indicate that higher grades (MF-2 and MF-3) of fibrosis correlate with defined clinical and molecular variables and independently negatively impact on OS in PMF, suggesting the opportunity to explore its value in the setting of clinical and molecular prognostic scores for PMF.
| . | Multivariate Analysis . | |||
|---|---|---|---|---|
| Variables | HR | 95% CI | P value | |
| HMR status | 2.4 | 1.5-3.7 | <.0001 | |
| HMR≥2mutations | 4.3 | 2.8-6.4 | .009 | |
| IPSS scoring | Int1 | 2.9 | 1.6-5.1 | <.0001 |
| Int2 | 10.0 | 5.6-17.7 | <.0001 | |
| High | 9.7 | 5.5-17.2 | <.0001 | |
| Driver mutations | CALR type2 | 3.4 | 1.3-8.6 | .010 |
| JAK2/MPL | 2.4 | 1.4-4.3 | .003 | |
| TN | 4.5 | 2.3-8.8 | <.0001 | |
| Fibrosis MF-2/MF-3 | 3.8 | 1.4-10.6 | .010 | |
| . | Multivariate Analysis . | |||
|---|---|---|---|---|
| Variables | HR | 95% CI | P value | |
| HMR status | 2.4 | 1.5-3.7 | <.0001 | |
| HMR≥2mutations | 4.3 | 2.8-6.4 | .009 | |
| IPSS scoring | Int1 | 2.9 | 1.6-5.1 | <.0001 |
| Int2 | 10.0 | 5.6-17.7 | <.0001 | |
| High | 9.7 | 5.5-17.2 | <.0001 | |
| Driver mutations | CALR type2 | 3.4 | 1.3-8.6 | .010 |
| JAK2/MPL | 2.4 | 1.4-4.3 | .003 | |
| TN | 4.5 | 2.3-8.8 | <.0001 | |
| Fibrosis MF-2/MF-3 | 3.8 | 1.4-10.6 | .010 | |
Passamonti:Novartis: Consultancy, Honoraria, Speakers Bureau. Barbui:Novartis: Speakers Bureau. Vannucchi:Shire: Speakers Bureau; Novartis: Other: Research Funding paid to institution (University of Florence), Research Funding; Baxalta: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract