Abstract
Introduction: PEGylation technology has been used to increase half-lives of therapeutic proteins, including clotting factors VIII and IX. However, there are concerns about the potential accumulation of PEG in hemophilia patients receiving PEGylated clotting factors chronically. Several studies have attempted to address these concerns by investigating the biodistribution of 14C or 3H-labeled PEGs alone or as PEGylated clotting factors in rats after a single high dose administration. The low specific radioactivity of 14C and 3H prevents the possibility of conducting these experiments at therapeutically relevant doses, and the relevance of supraphysiological dose levels, which likely lead to clearance through non-physiological pathways, is unclear. The objectives of this study were:
1. To develop a novel method to specifically label the PEG portion of a PEGylated rFVIII molecule with 125I, which has 50 times higher specific radioactivity than 3H
2. To determine the ultimate disposition of the PEG following therapeutically relevant single or repeat doses of 125I-labeled PEGylated rFVIII
Methods: To produce 125I-labeled PEGylated rFVIII, a 60 kDa branched PEG molecule having a strained cyclooctyne linker was first attached to the K1804C residue on a B-domain deleted human rFVIII via a maleimide linker. The 125I label was subsequently introduced by a selective reaction between an azide compound carrying the 125I label and the cyclooctyne linker. 125I-labeled PEG-cysteine (PEG control) and 125I-labeled FVIII without the 60kDa PEG (rFVIII) were also produced similarly to serve as controls. This final linker design was selected after extensive studies on multiple model linkers and was demonstrated by mass spectrometry to remain stable in plasma and liver homogenates.
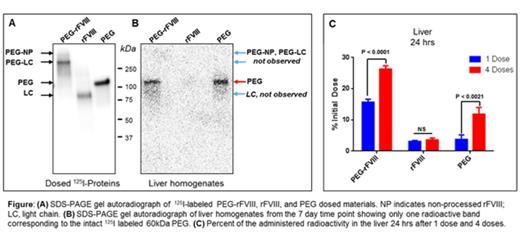
HemA mice received either single or repeat doses of either 80 IU/kg of 125I-labeled PEG-rFVIII or rFVIII, or equivalent radioactivity for the PEG control. In the single-dose group, selected organs were collected, perfused and radioactivity was measured at 15 min and 24 hrs post dose (n = 4, per time point). In the repeat-dose group, mice were dosed every 3 days for a total of 4 doses and radioactivity from selected organs was measured at 15 min, 24 hrs, and 7, 14 , and 28 days after the last dose (n = 3, per time point). Livers and spleens from the 7 day time point were homogenized and analyzed by autoradiography following SDS PAGE.
Results: rFVIII and the PEG moiety of PEGylated rFVIII were efficiently labeled with 125I without loss of FVIII activity (Fig. A). For the single-dose PEG control group, greater than 50% of administered radioactivity remained in the plasma after 24 hrs, while only 4 % was detected in the liver. In the rFVIII group, FVIII distributed quickly into organs, with the highest density in the liver and spleen; 15 min post injection, 18.8 % of administered radioactivity was detected in the liver, but was cleared after 24 hrs as expected. In contrast, the PEG-rFVIII group showed radioactivity increasing from 6.9 % at 15 min to 15.7 % at 24 hrs. The higher level at 24 hrs suggests that the rate of uptake exceeded the rate of liver metabolism, resulting in PEG accumulation. SDS-PAGE gels of liver and spleen homogenates extracted 7 days after the 4th dose of PEG alone or PEG-rFVIII, but not rFVIII, showed only one radioactive band corresponding to the intact 125I labeled 60kDa PEG, providing clear evidence of retention of PEG in these organs (Fig. B). Moreover, 24 hrs after the 4th dose, significantly higher levels of radioactivity were found in liver, spleen, and muscle (26.3 %, 2.9 %, 10.5 %) compared to those at 24 hrs after only one dose (15.7 %, 1.5 %, 3.1 %) (Fig. C). The radioactivity remained well detected in all organs after 28 days in mice dosed with PEG-rFVIII, with the highest level in the liver at 7.3 %.
Conclusions: A novel method that allows for studying the biodistribution of PEGylated rFVIII at therapeutically relevant doses was successfully developed. The 125I label was stable and fit for the purpose of following PEG metabolism and accumulation. Our results strongly suggest that the FVIII moiety dictates the biodistribution of PEG-rFVIII. However, while the FVIII portion is degraded, the PEG accumulates with repeat dosing (q3d) within various organs, particularly the liver and spleen. Further studies will be necessary to identify the specific cells within which the PEG accumulates, and the cumulative effects of prolonged dosing.
Hong:Biogen: Employment, Equity Ownership. Ismail:Biogen: Employment. Moore:Biogen: Employment, Equity Ownership. Peters:Biogen: Employment. Salas:Biogen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.