Abstract
Introduction
As of June 2015 the FDA has approved an alternative procedure under 21 CFR 640.120 that allows for storage of apheresis platelets at refrigerator temperature (1-6 C; 4°C) without agitation for up to 3 days for use in the resuscitation of actively bleeding patients. Understanding underlying mechanisms responsible for enhanced hemostatic function at 4°C will be critical for such improvements in platelet transfusion. We hypothesized that 4°C platelets display better mitochondrial respiratory function for up to 7 days compared to standard 5-day RT platelets and that mitochondrial gene expression differences between RT and 4°C -stored platelets will correlate with mitochondrial function.
Methods
Platelets were collected from healthy donors by apheresis according to an IRB-approved protocol. Apheresis platelets (AP) were rested for 1 h before allocation into platelet minibags (Blood Cell Storage, Seattle, WA) and stored for 4 storage durations (Baseline (BL), Day 3, 5, and 7). Mitochondrial respiration, maximal oxygen utilization, and individual mitochondrial complex-dependent respiration were assessed with high-resolution respirometry (O2k, Oroboros). Mitochondrial ROS generation in response to storage condition or stimulation (to assess oxidative burst capacity as a measure of function) was visualized with fluorescent imaging and assayed with flow cytometry using a superoxide stain (Life Technologies). Total RNA was extracted both immediately following apheresis (BL) and on Day 5 from RT and 4°C-stored platelets using Trizol (Molecular Research Center, Cincinnati, OH) after centrifuging the platelets at 900 x g for 10 min. Platelet RNA was quantified using the NanoDrop 2000. RNA quality was examined using gel electrophoresis with the Reliant Gel System (Cambrex, Rockland, ME). Platelet mitochondrial gene expression analysis was evaluated using the 96-well RT2 Profiler PCR Array (Qiagen, Valencia, CA) which profiled 84 mitochondria-focused targets and 12 control genes per sample. Gene expression data analysis was based on the ΔΔCt method with normalization of the raw data to housekeeping genes located on each 96-well plate.
Results
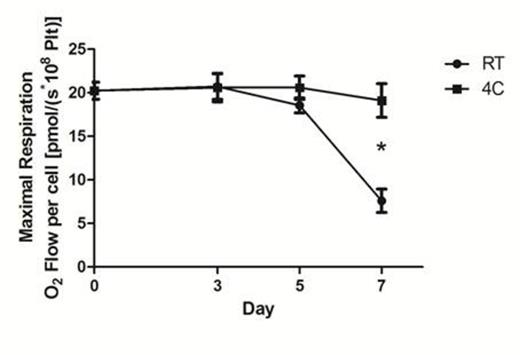
Mitochondrial respiration was lower in platelets stored at 4°C compared to RT on Days 3, 5, and 7 (Day 5= -57%±0.3; P < 0.05), demonstrating that refrigeration slows metabolism. Additionally, maximal mitochondrial oxygen utilization (electron transport system capacity) was better preserved in platelets stored at 4°C (Figure 1). Fluorescent imaging and flow cytometry demonstrated that mROS generation was higher in RT-stored platelets compared to 4°C, reflecting mitochondrial damage. Mitochondrial burst during de novo mROS generation due to stimulation was also preserved at 4°C. Mitochondrial gene expression studies revealed distinct differences in expression profiles for 4°C versus RT-stored platelets after 5 days of storage when normalized to BL measures. Storage at 4°C resulted in significantly greater preservation of 15 gene products at Day 5 (P<0.05). In contrast, Day 5 RT samples resulted in a marked decrease or loss of gene products when compared to BL levels of gene expression (P<0.05).
Discussion
Platelet mitochondrial respiratory function (mitochondrial respiration and maximal oxygen utilization) decreased in RT-stored platelets over 7 days, but the impairment was attenuated by 4°C storage. We previously noted that intracellular ROS flux was higher at room temperature, and here the gene expression analysis in combination with oximetry data showed that mitochondrial damage is likely responsible. Furthermore, gene expression profiling of mitochondrial-related genes revealed that distinct differences exist in key mitochondrial genes between the storage conditions. This work illustrates that 4°C storage of platelets preserved and enhanced critical mitochondrial genes compared to RT; this finding combined with improved mitochondrial respiratory measures and reduced ROS demonstrates a significant improvement in current efforts to mitigate platelet dysfunction.
Maximal respiration induced by titration of the protonophore FCCP (carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone) demonstrated a significant decrease in mitochondrial capacity (indicating loss of function) in RT-stored samples compared to 4 °C-stored samples by Day 7. Values are mean ± SD (n=7); *P<0.001 compared to RT.
Maximal respiration induced by titration of the protonophore FCCP (carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone) demonstrated a significant decrease in mitochondrial capacity (indicating loss of function) in RT-stored samples compared to 4 °C-stored samples by Day 7. Values are mean ± SD (n=7); *P<0.001 compared to RT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.