Abstract
Adipocytes are the prevalent stromal cell type in aged adult bone marrows (BM). We previously demonstrated prominent pro-survival role of BM-derived adipocytes for the acute monocytic leukemia (AMoL) cells, a poor-prognosis subtype of AML (Tabe ASH. 2013). A novel anticancer agent avocatin B, an odd-numbered carbon lipid derived from avocado fruit, has been shown to induce leukemia cell death by inhibiting fatty acid oxidation (FAO) via its accumulation in mitochondria (Lee, Cancer Res. 2015). In this study, we investigated the cytotoxic efficacy and molecular mechanisms of avocatin B in AMoL cells co-cultured with BM-derived adipocytes, mimicking the aging BM microenvironment. AMoL cell lines (THP1, MOLM13 and U937) and mesenchymal stem cells (MSC)-derived adipocytes were used for this study.
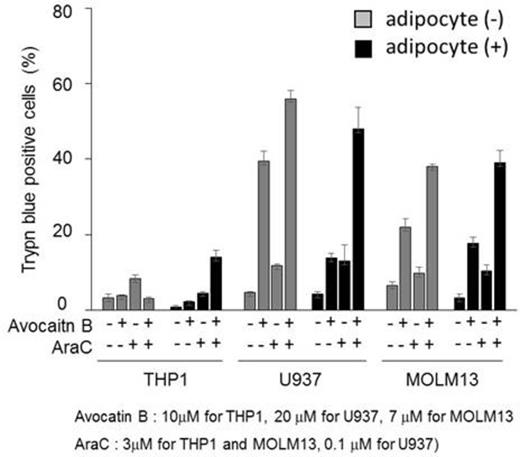
Adipocytes inhibited spontaneous apoptosis in AMoL cells, consistent with our prior observations. Avocatin B successfully induced apoptosis and cell growth inhibition in AMoL cells (IC50s between 15 and 73uM) with G0/G1 cell cycle accumulation. We further observed that avocatin B synergistically enhanced AraC induced apoptosis in AMoL cells cultured alone or co-cultured with adipocytes (Figure 1). To this end, avocatin B synergized with Ara C with combination index value of 0.15.
Immunoblot analysis demonstrated that avocatin B inactivated the stress response kinase phospho- (p-) AMPK and p-p38 MAPK in MOLM13 co-cultured with adipocytes but not in AML cells cultured alone. These results indicate that avocatin B disrupted the energy homeostasis under adipocyte co-culture condition.
Metabolic profiling using the capillary electrophoresis mass spectrometry (CE-MS) detected alteration of 12 polar metabolites (fold change > 2, P<0.05) in THP1 cells after adipocyte co-culture, including downregulation of Glucose 6-phosphate and Fructose 6-phosphate, and upregulation of citric acid, fumaric acid, malic acid and NAD+, which is consistent with AMPK signaling activation and suggests the downregulation of glycolysis and the compensatory activation of oxidative phosphorylation and FAO.
To further characterize the molecular mechanisms of pro-apoptotic effects of avocatin B, we focused on the gene transcriptional modulation induced by avocatin B in adipocyte co-cultures. We previously reported that CPT1(carnitine palmitoyltransferase I), a key enzyme of FAO, induced mitochondrial accumulation of avocatin B which resulted in AML cell apoptosis (Lee, Cancer Res. 2015). By RT-PCR analysis, we observed that avocatin B itself induced CPT1 (carnitine palmitoyltransferase I) mRNA. In addition, adipocyte co-culture upregulated FABP4 (fatty acid binding protein 4)which was further increased by avocatin B treatment in THP1, U937 and MOLM13 cells. These findings likely reflect the direct feedback of FAO inhibition by avocatin B.
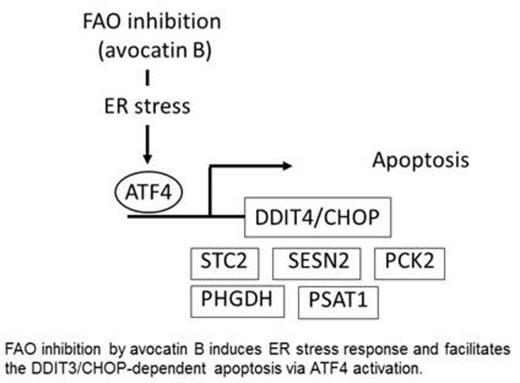
DNA microarray (Affymetrix) detected the upregulation of 45 genes and downregulation of 58 genes in THP1 cells after co-culture with adipocytes (> 2.0 fold). Ingenuity Pathway Analysis (IPA) and KEGG bioinformatics tools highlighted the cytokine-cytokine receptor interaction as the top upregulated pathway with the potent upstream regulators CXCL12, STAT3, p38 MAPK and NFkB activation. In turn, avocatin B treatment upregulated 71 genes and downregulated 27 genes in THP1 cells co-cultured with adipocytes. Among induced genes, avocatin B treatment caused upregulation of the stress response genes DDIT4, SESN2, PCK2, PHGDH, PSAT1 and STC2 that are the downstream targets of transcription factor ATF4, the master regulator of the endoplasmic reticulum (ER) stress response.
In summary, the avocatin B and AraC combination induced significant leukemia cell death under adipocyte co-culture conditions. Metabolome and transcriptome analyses indicate that FAO inhibition by avocatin B induced ER stress might stimulate the DDIT3/CHOP-dependent cell death via transcriptional activation of ATF4 (Figure 2). We conclude that the strategies targeting FAO warrant further exploration in patients with AMMoL, highly dependent on altered lipid metabolism.
Konopleva:Novartis: Research Funding; AbbVie: Research Funding; Stemline: Research Funding; Calithera: Research Funding; Threshold: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.