Abstract
Background
Inotuzumab ozogamicin (InO) is a targeted antibody-drug conjugate (ADC) under development for treatment of patients with relapsed or refractory CD22-positive acute lymphoblastic leukemia (ALL) and non-Hodgkin's Lymphoma. Correlative analyses from an open-label, Phase 1/2 study (B1931010) included exploring the relationship between InO pharmacokinetic exposure, hematologic measures, and gene expression to treatment response.
Methods
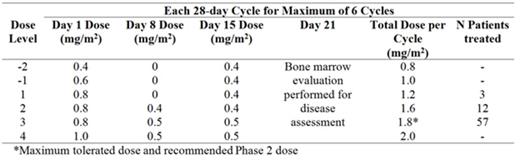
Patients received 2 to 3 weekly intravenous doses of InO by 1 hour infusion over a 28-day cycle for ≤ 6 cycles (table).
Blood samples were drawn during Days 1 and 15 of Cycles 1 and 2 (at 0, 1 and 3 h), and Day 1 of Cycle 4 (at 0 and 1 h) for concentration determination of InO and unconjugated calicheamicin in serum. Samples were analyzed using a validated LC/MS/MS procedure. InO levels were correlated to CD22 protein expression on CD19+ B-lymphocytes in blood and bone marrow, and to minimum residual disease. Lymphocyte regeneration was described using a linear mixed-effects model. To explore the relationship between clinical outcome and expression of genes such as those involved in DNA damage response and apoptosis, optional blood samples for gene expression analysis were collected on Cycle 1 Day 1 and Cycle 1 Day 15 [each at pre- and post-dose time-points]. These samples were assessed for baseline gene expression and gene expression changes using 96-gene TaqMan Low Density Array cards.
Results
Unconjugated calicheamicin levels were below the limit of quantitation (50 pg/mL) for most patients and time points. Treatment-related decreases in CD22 antigen expression on lymphocytes were rapid but unrelated to InO concentration. Lymphocyte depletion in blood was also rapid, consistently observed regardless of InO dose, and followed by slow regeneration with substantial inter-patient differences in regeneration rate. Percentage of bone marrow blasts was directly related to InO elimination rate. With treatment, as blast percentage decreased, InO elimination rate decreased by approximately 50% by Cycle 4 (ie, after 10 dosing events) compared to the first dose. In bone marrow, lower disease burden at baseline tended to be associated with faster regeneration of lymphocytes during the follow-up period. Patients achieving minimal residual disease (MRD)-negativity tended to have higher peak and trough serum concentrations of InO throughout each cycle compared to patients not achieving MRD-negativity. There was no observed correlation between percentage of blasts at baseline and MRD-negativity. CD22 mRNA levels in blood decreased approximately 10-fold by Day 15 relative to baseline, consistent with selective killing of CD22-positive leukemic blasts by InO. This decrease was more pronounced in subjects who exhibited complete response with/without incomplete platelet count recovery with no evidence of MRD than in subjects who did not exhibit clinical response (p=0.001 using Wilcoxon Rank Sum Test). In addition, multiple other transcripts exhibited decreases following InO administration, including mRNAs encoded by genes regulating proliferation (cyclin-dependent kinase 2), DNA repair (XRCC2), and cell death (death-associated protein kinase 1).
Conclusion
In patients with ALL receiving InO, CD22 protein expression and lymphocyte count decreased rapidly, followed by slow and variable regeneration, as patients with low disease blast counts at baseline exhibited faster recovery of their counts than those with higher levels. While CD22 expression is not a significant determinant of InO concentration, the number of doses administered did influence drug elimination rate; an effect thought to be associated with target-mediated drug clearance. In related fashion, patients achieving MRD-negativity tended to have higher InO concentrations following InO treatment. Changes in mRNA profiles in blood consistent with the mechanism of action of InO were evident. The extent of reduction in CD22 mRNA was associated with clinical outcome. Study is ongoing for long term follow up.
Fostvedt:Pfizer Inc: Employment, Other: stock ownership. Laird:Pfizer Inc: Employment, Other: Stock ownership. Marshall:Pfizer Inc: Employment, Other: Stock ownership. Li:Pfizer Inc: Employment, Other: Stock ownership. Boni:Pfizer Inc: Employment, Other: Stock ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract