Abstract
Introduction: SLIT-ROBO is newly discovered ligand-receptor family of neuronal guidance molecules. Recently, it has been proved that these proteins are involved in both, physiologic and pathologic angiogenesis. In animal models, it was shown both, pro-and antiangiogenic of SLIT-ROBO signaling. Moreover, the interaction of SLIT ligands with their roundabout receptors (ROBO) results in promotion of apoptosis, adhesion and blocking of cell cycle. There is evidence that SLIT-ROBO proteins are involved in pathogenesis of solid tumors, both in angiogenesis dependent and independent way. The role of SLIT-ROBO proteins in biology of acute myeloid leukemia (AML) remains unknown.The only two hitherto published studies considering ROBO4 expression in AML have revealed its increased expression in the blasts cells.
The aim of the study was to evaluate the role of SLIT-ROBO proteins in AML. The expression of SLIT ligands, and their receptors ROBO was assessed in bone marrow of newly diagnosed AML patients and in the control group. The expression level of the proteins was correlated with known prognostic factors, response to treatment and overall survival (OS), as well as angiogenesis activity.
To our knowledge, it has been the first study investigating the whole family of SLIT-ROBO proteins in AML.
Methods: Expression SLIT-ROBO proteins was assessed in bone marrow biopsy specimens of 79 newly diagnosed AML patients with median age 59 years [18-87]. The paraffin-embedded tissue blocks were retrieved and subjected to immunohistochemistry for SLIT ligands (SLIT1, SLIT2, SLIT3), and their receptors ROBO1, ROBO2, ROBO3, and ROBO4. The positive blasts cells were semi-quantitatively analyzed according to previously published methods (Perrone et al, 2006). For the purpose of analysis the patients were divided into "low-expressers" and "high-expressers". Concurrently, all samples were immunostained for CD34 to calculate microvessel density (MVD) as an equivalent of angiogenesis. The control group was composed of 23 BM biopsies form patients with newly diagnosed lymphoma without bone marrow involvement.
Results:
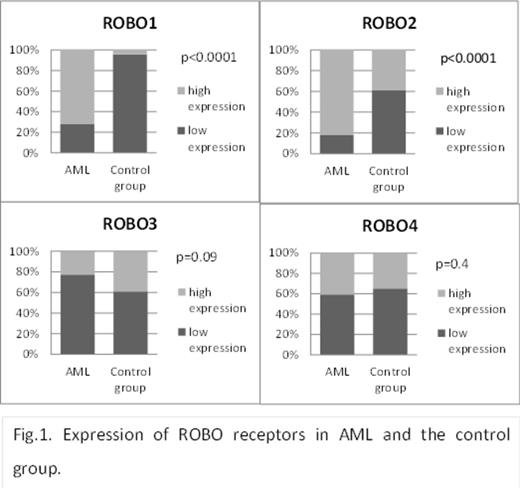
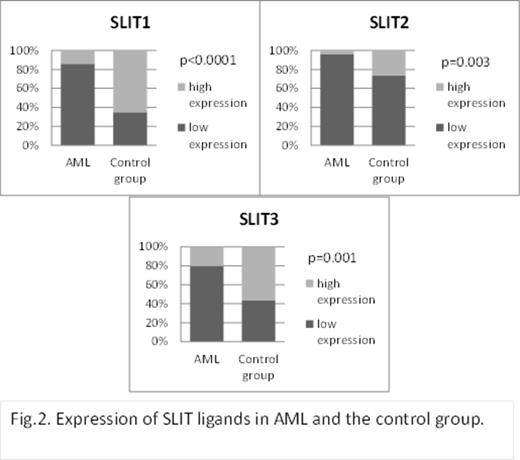
Expression of ROBO receptors and SLIT ligands in AML patients and in the control group.
In our study higher expression of ROBO1, ROBO2, and ROBO3 was observed more often in AML patients compared to the control group (p<0.0001, p<0.001, and p=0.09, respectively, Fig 1.). In contrast, low expression of SLIT1, SLIT2, and SLIT3 ligands has been shown more often in AML than in control BM samples (p<0.0001, p=0.003, and p=0.001, respectively,Fig.2.). Higher expression of ROBO1, ROBO2, and ROBO3 was more often in AML patients ≥60 years (p=0.04, p=0.008, and p=0.02, respectively).Conversely, low expression of ROBO4 was more often observed in elderly AML (p=0.06). The majority of patients with de novo AML had low expression of SLIT1 and SLIT2 (p=0.053 and p=0.055, respectively). As to ROBO, higher expression of ROBO2 in the group with secondary AML was more frequent (p=0.09). No significant correlations between the SLIT-ROBO proteins' expression,neither cytogenetic risk group nor clinical stage parameters such as WBC, hemoglobin level, proportion of leukemic blasts in BM, or LDH activity were found. Similarly, neither of the SLIT-ROBO proteins influenced the complete remission rate (CR) and overall survival (OS).
Relationship between SLIT-ROBO expression and angiogenesis activity in AML patients and control group.
Significantly higher MVD in BM of AML patients than in control group (Me 51 [9-140] vs 16 [4-78], p<0.0001) has been observed. ROBO4was the only protein that expression correlated significantly with MVD. Higher expression of ROBO4 was associated with higher MVD in both, AML and the control group (p=0.05 and p=0.01, respectively).
Conclusions:
SLIT-ROBO family members play a role in biology of AML. ROBO4 is involved in both, physiologic and pathologic angiogenesis A better understanding of SLIT-ROBO signaling pathway in leukemic blasts may create new optionsfor AML therapy.
Acknowledgments:
AG and DJ-K both equally contributed to the study.
This work was supported by grants from Medical University of Lodz, Lodz, Poland (502-03/1-093-01/502-14-077 and 503/1-093-01/503-11-001).
Robak:Eisai Inc: Research Funding. Wierzbowska:Janssen, Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.