Abstract
Introduction
MRI is the imaging gold standard method for the detection of bone marrow involvement in multiple myeloma (MM) (Dimopoulos. J Clin Oncol.2015). MRI of the spine and pelvis can detect approximately 90% of focal lesions in MM and is the procedure of choice to evaluate a painful lesion in myeloma patients, mainly in the axial skeleton. It has also been reported that in patients treated with frontline autologous stem cell transplantation (ASCT) the number of MRI focal lesions and the presence of diffuse pattern correlate with inferior survival (Walker et al. J Clin Oncol.2007). MRI might also help in the better definition of complete response (CR). However, the high number of false positive results suggests that another imaging method, such as Positron Emission Tomography combined with computed tomography using fluoro-deoxy-glucose (PET-CT), might be of more value in this setting (Zamagni. Clin Cancer Res.2015). The goal of our study was to compare prospectively MRI and PET-CT at 3 different time-points, at diagnosis, after 3 cycles of triplet induction therapy and prior to maintenance therapy in a group of patients enrolled into IFM-DFCI 2009 trial comparing frontline or delayed ASCT.
Patients and methods
700 patients with de novo symptomatic MM eligible for high-dose therapy (HDT) have been prospectively randomized in France and Belgium to receive either 8 cycles of bortezomib-lenalidomide-dexamethasone (VRD) followed by 1-year maintenance with lenalidomide, or 3 cycles of VRD followed by HDT and ASCT plus 2 cycles of VRD consolidation and 1-year lenalidomide maintenance. 134/700 patients were also included in the IMAJEM trial (NCT01309334) aimed at comparing in both arms of the IFM-DFCI 2009 study spine and pelvis MRI and whole-body PET-CT at diagnosis (number of lesions, primary end-point), after 3 cycles of VRD, and prior to maintenance (prognosis impact of imaging normalization, secondary end-point). MRI and PET-CT data were analyzed locally in each of the 15 participating centers, and systematically reviewed blindly by an independent committee consisting of 2 radiologists and 2 nuclear medicine physicians with extensive experience in MM field.
Results
At diagnosis, MRI was positive in 127/134 (94.7%), and PET-CT in 122/134 (91%) patients, respectively (McNemar test = 0.94, p = 0.33).
Following 3 cycles of VRD, MRI remained positive in 93%, and PET-CT in 55% of the patients, respectively. Normalization of MRI after 3 months of induction therapy was not predictive neither for progression-free survival (PFS) (p = 0.99) nor for overall survival (OS) (p = 0.99). Normalization of PET-CT after 3 months of induction therapy was associated with a significant improvement in PFS (30-month PFS rate: 60% in patients PET-CT positive vs 79% in patients PET-CT negative, p = 0.04), but not OS (30-month OS rate: 82 % in patients PET-CT positive vs 93 % in patients PET-CT negative, p = 0.15).
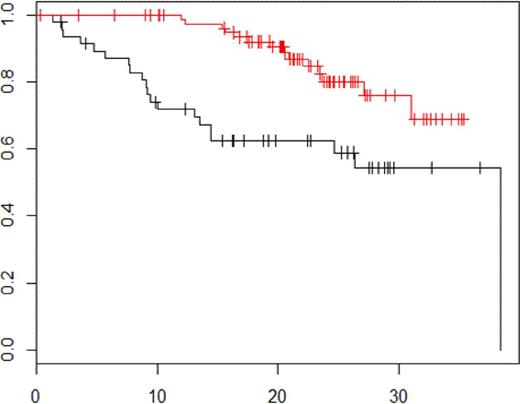
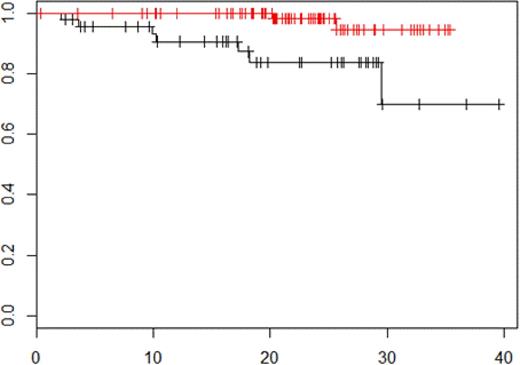
Prior to maintenance therapy, MRI remained positive in 83%, and PET-CT in 21% of the patients, respectively. Normalization of MRI before maintenance was not predictive neither for PFS (p = 0.31) not for OS (p = 0.98). Normalization of PET-CT before maintenance was associated with a significant improvement in both PFS (30-month PFS rate: 54.4% in patients PET-CT positive vs 75.9% in patients PET-CT negative, p = 0.0004, figure 1) and OS (30-month OS rate: 69.9% in patients PET-CT positive vs 94.6% in patients PET-CT negative, p = 0.01, figure 2).
Conclusion
MRI of the spine and pelvis and whole-body PET-CT are equally effective to detect bone involvement at diagnosis. Few patients achieved normal MRI after 3 cycles of VRD and before maintenance. Normalization of MRI following 3 cycles of VRD and before maintenance has no prognostic value for both PFS and OS. 45% of the patients achieved normal PET-CT following 3 cycles of VRD and 79% of the patients achieved normal PET-CT before maintenance. Normalization of PET-CT following 3 cycles of VRD and before maintenance was associated with a significant improvement for PFS. Normalization of PET-CT before maintenance was a predictor for an improved OS. PET-CT should be incorporated in the follow-up of young patients receiving novel-agent based therapy to predict outcome.
PFS according to PET-CT negativity vs PET-CT positivity before maintenance therapy p = 0.0004.
PFS according to PET-CT negativity vs PET-CT positivity before maintenance therapy p = 0.0004.
OS according to PET-CT negativity vs PET-CT positivity before maintenance therapy p = 0.01.
OS according to PET-CT negativity vs PET-CT positivity before maintenance therapy p = 0.01.
Moreau:Celgene, Janssen, Takeda, Novartis, Amgen: Membership on an entity's Board of Directors or advisory committees. Attal:celgene: Membership on an entity's Board of Directors or advisory committees; jansen: Honoraria. Karlin:Celgene: Honoraria; Janssen: Honoraria; BMS: Honoraria; Amgen: Honoraria; Sandoz: Honoraria, Membership on an entity's Board of Directors or advisory committees. Facon:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees; Onyx: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Pierre Fabre: Membership on an entity's Board of Directors or advisory committees. Stoppa:Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding. Laribi:Hospira SAS: Research Funding. Hulin:Bristol Myers Squibb: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Celgene Corporation: Honoraria. Richardson:Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium Takeda: Membership on an entity's Board of Directors or advisory committees; Gentium S.p.A.: Membership on an entity's Board of Directors or advisory committees, Research Funding. Anderson:Celgene: Consultancy; Gilead: Consultancy; BMS: Consultancy; Millennium: Consultancy; Acetylon: Equity Ownership; Oncopep: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract