Abstract
Background
Minimal residual disease (MRD) response after first-line treatment of follicular lymphoma (FL) is likely to predict clinical course. The prognostic relevance of MRD in relapsed/refractory (r/r) FL remains unclear. We report MRD assessment with respect to clinical outcomes in r/r FL pts in GADOLIN (NCT01059630). GADOLIN is an open-label, multicenter, randomized phase III study of pts with r/r indolent NHL (refractory to rituximab) to investigate the efficacy and safety of obinutuzumab (G) plus bendamustine (B) followed by G maintenance vs B alone. A significant improvement in PFS in the G-B arm was reported (Sehn L, et al. ASCO 2015).
Methods
MRD was analyzed by t(14;18) and/or Ig variable domain allele-specific RQ-PCR in pts with a clonal marker detectable at diagnosis in peripheral blood (PB) or bone marrow (BM) by consensus PCR. (Assessment of Ig rearrangements allows detection of a lymphoma marker in pts with a t(14;18) breakpoint not detected by generic PCR and avoids false positive signals, as a low level of the translocation can be detected in some healthy individuals.) Assays were designed with a sensitivity of 10-5 and accepted for MRD assessment when a sensitivity of ≤10-4 was reached. Results were evaluated according to ESG-MRD criteria. MRD status was analyzed at interim staging (C4, D1) and after end of induction (EOI), and defined as negative (-ve) if RQ-PCR and subsequent nested PCR produced a -ve result, i.e. achieving an MRD response. PFS was measured from the EOI date.
Results
321 of 396 randomized pts were diagnosed with FL. Baseline samples (PB and/or BM) were available for 285 of the 304 FL pts who had completed induction at the clinical cut-off date (1 Sep 2014; FL-ITT population). A clonal marker was detected in 183 (64%) of these pts; 128/183 (70%) had a RQ-PCR assay fulfilling the sensitivity criteria. EOI samples were available for 93 pts (biomarker-evaluable population) and 64 had a PB sample at mid-induction to assess MRD-response kinetics. The distribution of age, stage, and FLIPI in the biomarker-evaluable population was similar to the non-evaluable population of the B arm, while there was an enrichment of younger age, advanced-stage disease, and high risk FLIPI in the biomarker-evaluable population of the G-B arm.
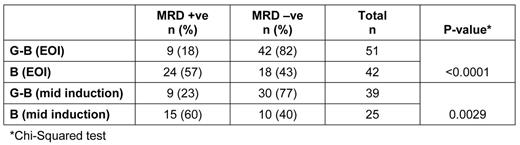
MRD response was analyzed in 93 pts at EOI and was significantly higher in pts receiving G-B induction, with 42/51 (82%) achieving MRD -ve status compared with 18/42 pts (43%) in the B arm (p<0.0001, Chi-Squared). At mid-induction, 30/39 (77%) pts in the G-B arm achieved early MRD -ve status vs 10/25 (40%) in the B arm (p=0.0029; Table). MRD response was associated with clinical CR; 2/33 (6%) MRD positive (+ve) pts vs 17/60 (28%) MRD -ve pts achieved a CR. Moreover, 39/63 pts with partial remission were MRD -ve.
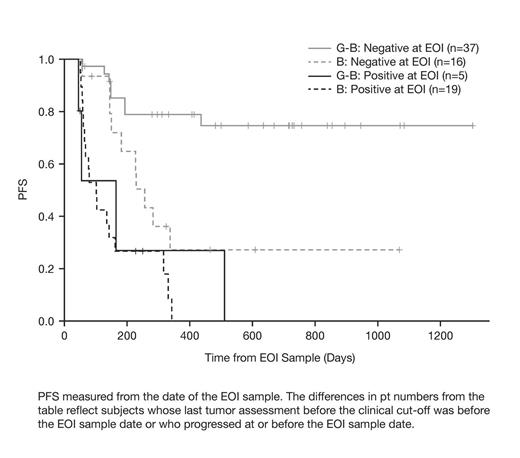
Pts in the G-B arm who were MRD -ve at EOI had an improved PFS at 24 mo post-EOI (74%; median PFS not reached) compared with the B arm (21%; median PFS, 7.6 mo). PFS for MRD non-responders was comparably poor in both treatment arms; all pts progressed before 24 mo with a post-EOI median PFS of 5.4 mo (G-B) and 3.0 mo (B; Figure).
Discussion
Our results suggest that G significantly contributes to the depth of response to B vs B alone during induction treatment and support the notion that MRD status at EOI treatment is a sensitive marker of efficacy in the setting of r/r FL. MRD response identifies a prognostically favorable group of pts that appear to benefit from treatment with G-B at relapse. The improved PFS outcome suggests that these pts also benefit from G maintenance. Pts without an MRD response had a very poor prognosis, irrespective of treatment arm. Future analyses will assess MRD kinetics during maintenance and follow-up.
PFS from the Date of the EOI Sample, by Treatment Arm and MRD Status
Off Label Use: Obinutuzumab (GA101; Gazyva/Gazyvaro) is a CD20-directed cytolytic antibody and is indicated, in combination with chlorambucil, for the treatment of patients with previously untreated chronic lymphocytic leukemia. This abstract reports on bendamustine with or without obinutuzumab in patients with CD20+ R-ref, indolent non-Hodgkin lymphoma.. Belada:Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Danesi:Roche: Employment. Fingerle-Rowson:Roche: Employment, Equity Ownership. Gribben:Celgene: Consultancy, Honoraria; Gilead: Honoraria; Roche/Genentech: Honoraria; Pharmacyclics: Honoraria; Janssen: Honoraria. Harbron:Roche: Employment, Equity Ownership; AstraZeneca: Equity Ownership. Kahl:Roche/Genentech: Consultancy; Seattle Genetics: Consultancy; Millennium: Consultancy; Cell Therapeutics: Consultancy; Celgene: Consultancy; Infinity: Consultancy; Pharmacyclics: Consultancy; Juno: Consultancy. Mundt:Roche/Genentech: Employment. Sehn:Roche/Genentech: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria. Cheson:Teva: Research Funding; Gilead: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Roche/Genentech: Consultancy, Research Funding; Spectrum: Consultancy; Astellas: Consultancy; Ascenta: Research Funding; AstraZeneca: Consultancy; MedImmune: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.